010 Phosphates, Sulfones and Sulfonates
Learning Outcomes
Apply nomenclature and structure determination of phosphates, sulfones and sulfonates;
Identify common reactions of phosphates, sulfones and sulfonates;
Explain the role and function of phosphates, sulfones and sulfonates in biological systems; and
Relate the importance of phosphates, sulfones and sulfonates to agricultural practices.
Phosphates, Sulfones and Sulfonates in Daily Life and Agriculture
Phosphates, sulfones, and sulfonates play essential roles in everyday life and are invaluable to agricultural practices. Phosphates are found in many foods as preservatives, stabilizers, and leavening agents, ensuring the freshness and texture of processed items. Sulfones, particularly in pharmaceuticals, treat conditions like leprosy and malaria, while sulfonates act as powerful surfactants in household cleaners and personal care products, aiding in stain removal and effective cleansing.
In agriculture, these compounds are indispensable for promoting plant growth, improving soil health, and protecting crops. Phosphates are especially crucial in fertilizers, supplying phosphorus, a key nutrient that fuels energy transfer within plants and supports root development, flowering, and fruiting. However, phosphates in natural soil are often in forms that plants cannot readily use, so fertilizers ensure plants receive adequate phosphorus, significantly boosting yields and crop quality.
Sulfones contribute indirectly to agriculture as intermediates in producing herbicides and pesticides. These sulfone-based agrochemicals help control pests and diseases, minimizing crop loss and improving productivity. Their stability also ensures a sustained effect, reducing the frequency of applications and lowering environmental impact.
Sulfonates enhance water infiltration and distribution when used in soil conditioners, allowing plants to absorb water more effectively. They also improve the spread and effectiveness of herbicides and pesticides on crops, optimizing agricultural input and reducing runoff.
Overall, phosphates, sulfones, and sulfonates support sustainable agricultural practices by enhancing nutrient availability, protecting crops, and improving water management—ultimately ensuring reliable food production to meet global demands.
Structure and Properties of Phosphates
Phosphates are essential compounds in both inorganic and organic chemistry, particularly due to their roles in biological systems and various industrial applications. This overview covers their structure, classification, and relevant physical and chemical properties, specifically for organophosphates.
Phosphates are characterized by the phosphate ion, which has the formula PO43−. Phosphates can be classified into several categories based on their structure and the number of phosphorus atoms, one of them being organophosphates. In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure O=P(OR)3, a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid. Organophosphates are best known for their use as pesticides.
Organophosphates are characterized by the presence of a phosphorus atom bonded to organic groups and oxygen. The general structure can be represented as:
where R1, R2, R3 represent organic groups. This structure allows for a variety of derivatives, leading to a diverse range of organophosphate compounds. They can be classified into several categories based on their specific chemical structures and uses:
Insecticides: Compounds like chlorpyrifos and diazinon are used extensively in agriculture to control pests.
Herbicides: Some organophosphates act as herbicides, targeting unwanted plants.
Nerve Agents: Certain organophosphates were developed for military use as chemical weapons (e.g., sarin).
Flame Retardants: Organophosphate esters are used in plastics to reduce flammability.
Industrial Applications: They serve as plasticizers, hydraulic fluids, and additives in lubricants.
Classification
Organophosphates are a class of compounds encompassing a number of distinct but closely related function groups. These are primarily the esters of phosphoric acid and can be mono‑esters, di‑esters or tri‑esters depending on the number of attached organic groups (abbreviated as 'R' in the image below). In general man‑made organophosphates are most often triesters, while biological organophosphates are usually mono- or di-esters. The hydolysis of triesters can form diesters and monoesters.
Physical Properties
State: Organophosphates can exist as liquids or solids at room temperature, depending on their molecular structure.
Solubility: Many organophosphates are soluble in organic solvents but have varying solubility in water.
Volatility: Some organophosphate compounds are volatile, which can lead to environmental concerns due to their potential for air pollution and contamination.
Chemical Properties
Cholinesterase Inhibition: A key feature of organophosphates is their ability to inhibit acetylcholinesterase (AChE), an enzyme essential for breaking down the neurotransmitter acetylcholine at synapses. This inhibition leads to an accumulation of acetylcholine, resulting in overstimulation of nerves, which can cause paralysis or death in insects and potential toxicity in humans25.
Hydrolysis: Organophosphates can undergo hydrolysis reactions, which can affect their persistence in the environment. The rate of hydrolysis varies significantly among different organophosphate compounds.
Reactivity with Nucleophiles: Organophosphates readily react with nucleophiles due to the electrophilic nature of the phosphorus atom, allowing them to form stable bonds with biological molecules.
Structure and Properties of Sulfones and Sulfonates
Sulfones and sulfonates are important classes of organosulfur compounds with distinct chemical structures and properties. They play significant roles in various applications, including pharmaceuticals, industrial processes, and materials science.
Sulfones are characterized by the presence of a sulfonyl group (RR'S(=O)2), where sulfur is bonded to two carbon atoms and double-bonded to two oxygen atoms. The general formula for a sulfone can be represented as:
where R and R' are typically hydrocarbon groups.
Sulfonates contain a sulfonic acid group (SO3H) attached to an organic moiety (In organic chemistry, a moiety is a part of a molecule that is given a name because it is identified as a part of other molecules as well). They are a salt or ester of a sulfonic acid. The general formula can be represented as:
| Sulfonate ion |
where R is an organic group. When deprotonated, the sulfonate ion carries a negative charge.
Physical Properties
Polarity and Hydrogen Bonding: Sulfones are polar compounds due to the presence of the sulfonyl group, which creates significant dipole moments. Sulfonates are also polar due to the presence of the sulfonic acid group. The strong electronegativity of sulfur and oxygen creates significant dipole moments, contributing to their polar nature.
Solubility: Sulfones are generally soluble in polar solvents such as water and alcohols. Sulfonates are also highly soluble in water because they can form hydrogen bonds with water molecules and interact with the polar solvent effectively. This solubility is enhanced by their ionic nature when deprotonated (forming sulfonate ions).
State: Sulfones and sulfonates can exist in both liquid and solid states at room temperature.
Chemical Properties
Sulfones are relatively inert compared to other organosulfur compounds, exhibiting lower oxidizing ability and higher acidity than sulfoxides. In reactions like the Ramberg–Bäcklund reaction and Julia olefination, sulfones can be converted to alkenes through the elimination of sulfur dioxide. They can undergo desulfonylation, leading to the formation of other compounds.
Sulfonic acids are strong acids, making sulfonates highly reactive in acid-base reactions. Sulfonate esters can participate in nucleophilic substitution reactions, where a nucleophile replaces the sulfonate group.
IUPAC Nomenclature of Organophosphates, Sulfones and Sulfonates
Naming Rules
Organophosphate:
- Base Name: The base name is derived from "phosphate," indicating that the compound is an ester of phosphoric acid.
- Substituent Naming: The alkyl or aryl groups attached to the phosphorus atom are named in alphabetical order, followed by "phosphate." For example: For a compound with two ethyl groups and one methyl group, the name would be diethyl methyl phosphate. If there are different substituents, such as a phenyl group and two butyl groups, it would be named dibutyl phenyl phosphate.
Diethyl methyl phosphate - Numbering: In cases where there is potential ambiguity regarding the position of substituents, numbering may be used to specify their locations relative to the phosphorus atom.

2-Ethylhexyl diphenyl phosphate
Sulfones:
- Base Name: The base name for sulfones is derived from the term "sulfone," which indicates the presence of the sulfonyl functional group.
- Substituents: The organic groups (substituents) attached to the sulfur atom are named first. These groups are listed in alphabetical order, followed by the term "sulfone." For example: If a sulfone has two methyl groups, it would be named dimethyl sulfone. For a compound with a phenyl group and butyl groups, it would be named butyl phenyl sulfone.
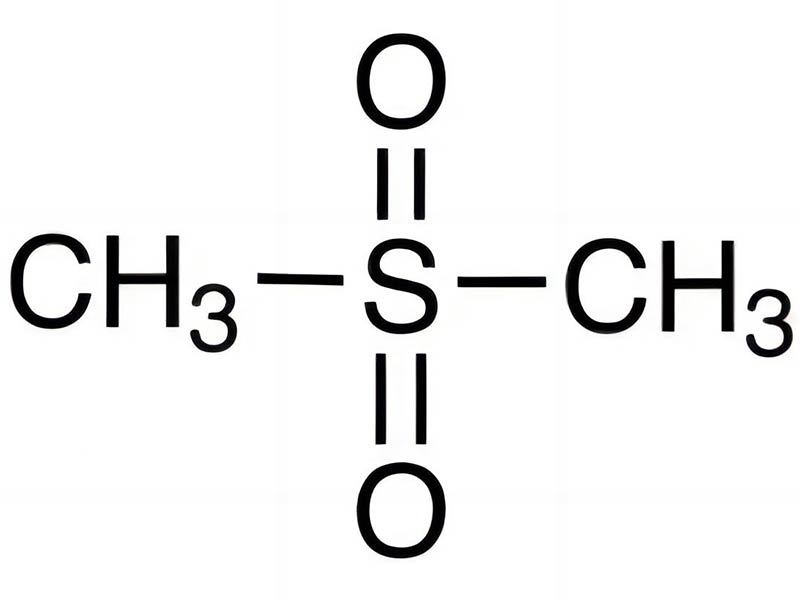
Dimethyl sulfone 
Butyl phenyl sulfone - Use of Prefixes: The prefixes "di-", "tri-", etc., are used to indicate the number of identical substituents.
Sulfonates:
- Base Name: Start by identifying the main hydrocarbon or organic group to which the sulfonate group is attached. In other words, the parent name will be based on the substituent attached to the sulfur atom. This will serve as the "parent" or base part of the name.
- Substituents: The organic groups (substituents) attached to the sulfur atom are named first. These groups are listed in alphabetical order, followed by the term "sulfonate." For example: A compound with a methyl group and a phenyl group would be named methyl benzenesulfonate. If there are two identical substituents, such as two ethyl groups, it would be named diethyl sulfonate.

Methyl benzenesulfonate
Problem Set
I. Draw the line notation for the following compounds based on their IUPAC names:
- Trimethyl phosphate
- Triethyl phosphate
- Dimethyl propyl phosphate
- Methyl dipropyl phosphate
- Tripropyl phosphate
- Diethyl propyl phosphate
- Butyl ethyl methyl phosphate
- Isopropyl methyl pentyl phosphate
- Dihexyl phenyl phosphate
- Triphenyl phosphate
- Diethyl sulfone
- Ethyl methyl sulfone
- Ethyl propyl sulfone
- Dipropyl sulfone
- Butyl propyl sulfone
- Dibutyl sulfone
- Hexyl pentyl sulfone
- Isopropyl pentyl sulfone
- Pentyl phenyl sulfone
- Diphenyl sulfone
- Methyl methanesulfonate
- Ethyl methanesulfonate
- Methyl ethanesulfonate
- Ethyl ethanesulfonate
- Propyl methanesulfonate
- Propyl ethanesulfonate
- Propyl propanesulfonate
- Butyl pentanesulfonate
- Isopropyl butanesulfonate
- Pentyl benzenesulfonate








.png)
.png)
.png)
Comments
Post a Comment