009 Amines and Amides
Learning Outcomes
Apply nomenclature and structure determination of amines and amides;
Identify common reactions of amines and amides;
Explain the role and function of amines and amides in biological systems; and
Relate the importance of amines and amides to agricultural practices.
Amides and Amines in Daily Life and Agriculture
Amines and amides are widely utilized in daily life and agriculture, thanks to their diverse chemical properties and biological roles. Amines, for example, are key building blocks in many everyday products, such as pharmaceuticals, dyes, and cleaning agents. In medicine, amines form the backbone of many drugs, including antihistamines and antidepressants, which are essential for treating various health conditions. Additionally, amines are found in household items like disinfectants and hair dyes, where they contribute to the product's effectiveness through their reactivity and solubility properties.
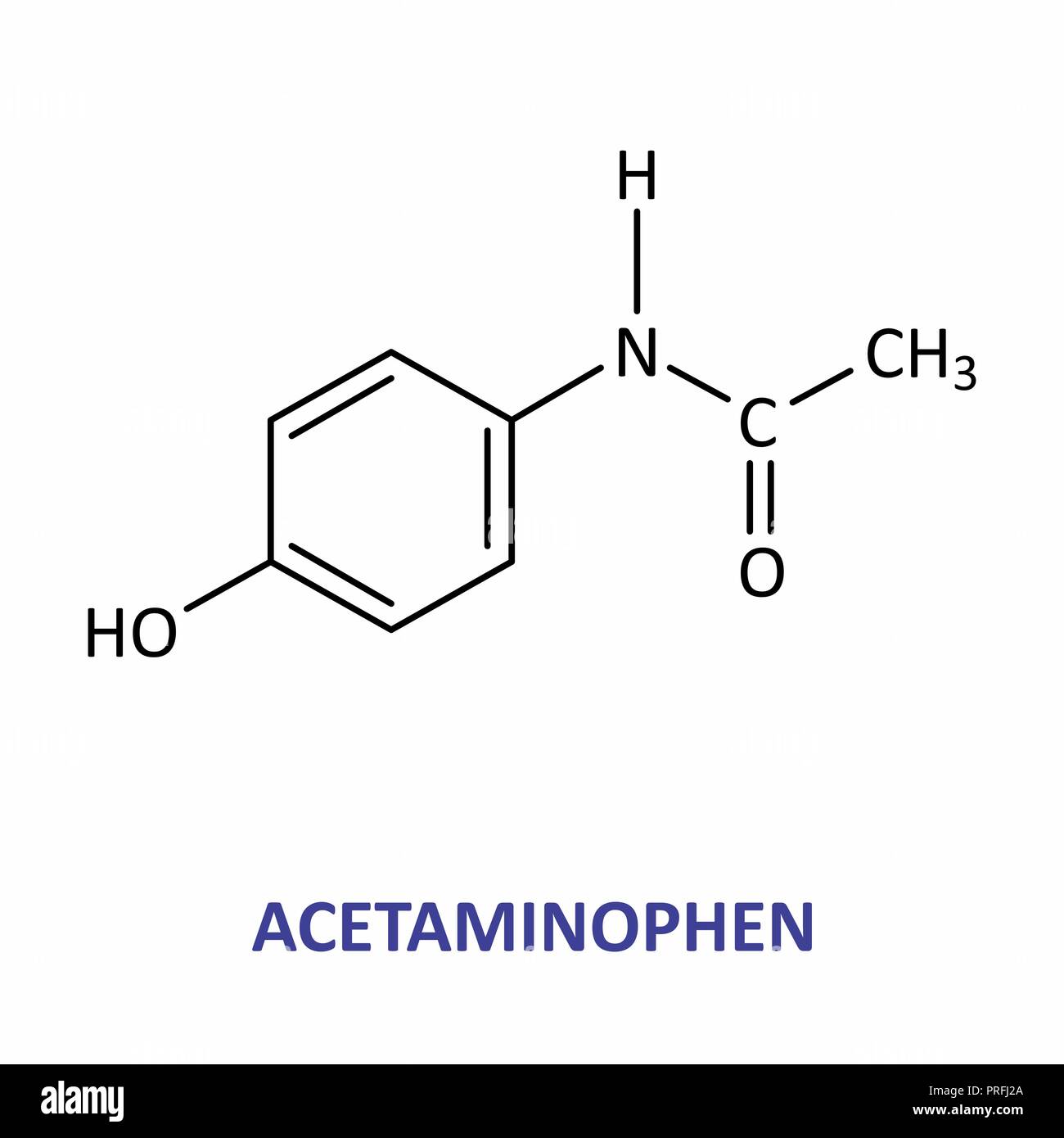 |
| Acetaminophen or paracetamol is an amide commonly used to treat fevers and as pain reliever. |
In agriculture, amides are critical components in fertilizers, particularly urea, which is a nitrogen-rich amide compound. Urea fertilizers are a major nitrogen source for crops, promoting rapid growth and higher yields by releasing ammonia into the soil, which plants can absorb. Certain amide herbicides, like propanil, help control weed growth by targeting specific plant enzymes, reducing competition for resources and enhancing crop health. Together, amines and amides play indispensable roles in maintaining plant health, protecting crops from pests, and supporting various household and medical applications.
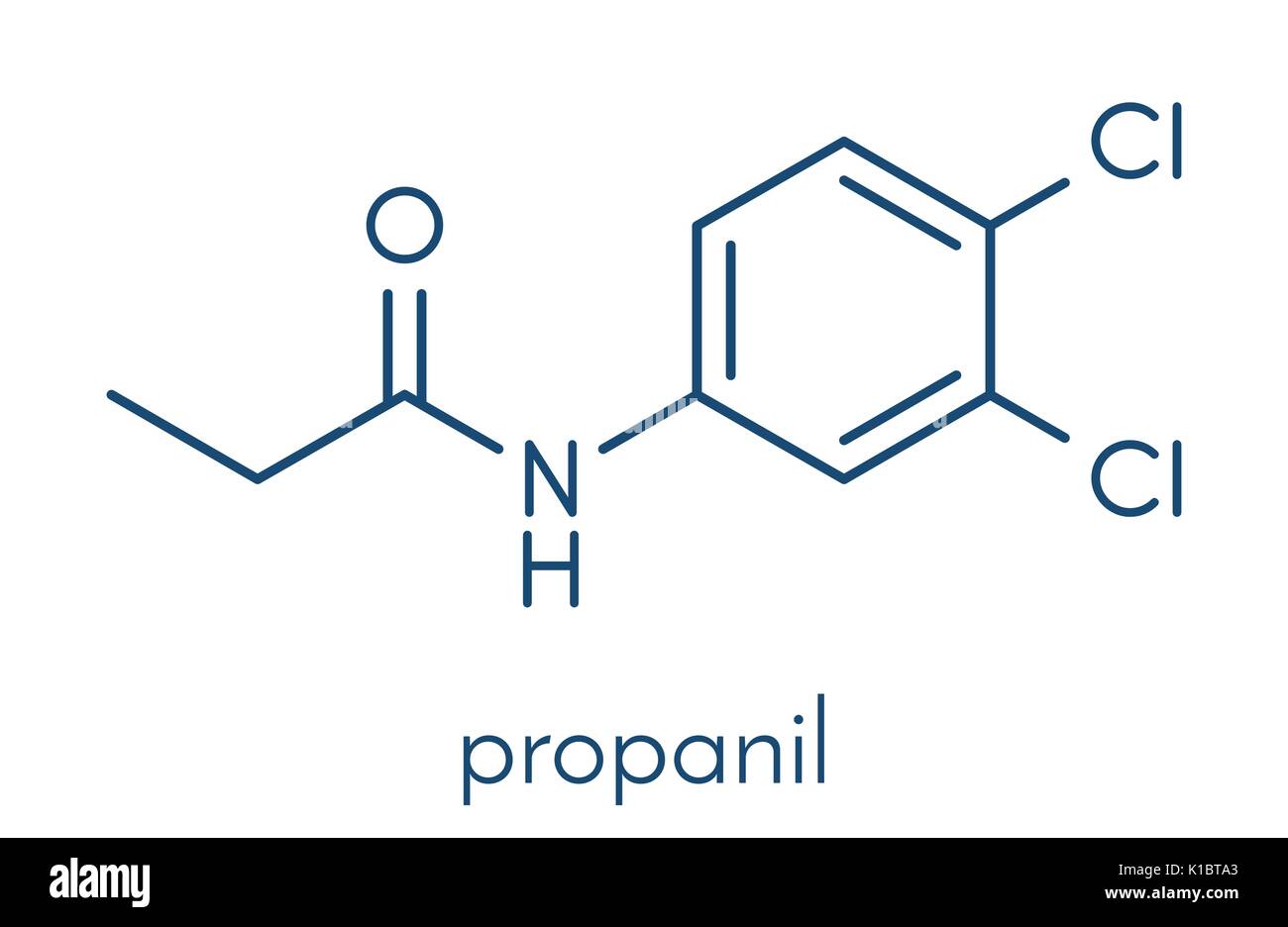 |
| Propanil, an amide, is used as a herbicide. |
 |
| Urea is an amine commonly used as a fertilizer. |
Structure and Properties of Amines
Amines are organic compounds derived from ammonia (NH₃) in which one or more hydrogen atoms are replaced by alkyl or aryl groups. They are essential in agriculture due to their roles as precursors in fertilizers, pesticides, herbicides, and plant growth regulators. The general structure can be represented as R-NH₂ (primary), R₂NH (secondary), or R₃N (tertiary), where “R” represents an organic group.
Classification
Primary Amines (1°): Nitrogen is bonded to one carbon group (R-NH₂)
Secondary Amines (2°): Nitrogen is bonded to two carbon groups (R₂NH).
Tertiary Amines (3°): Nitrogen is bonded to three carbon groups (R₃N).
Physical Properties
Solubility: Low molecular weight amines (e.g., methylamine, ethylamine) are generally soluble in water due to their ability to form hydrogen bonds with water molecules. Higher molecular weight amines are less soluble due to their larger nonpolar alkyl groups.
Polarity and Hydrogen Bonding: The nitrogen atom in amines has a lone pair of electrons, which contributes to its basicity and polarity. The electronegativity of nitrogen (3.04) is lower than that of oxygen, but it still creates a significant dipole moment when bonded to hydrogen atoms. Primary and secondary amines can form hydrogen bonds with water molecules due to the presence of N-H bonds, making them relatively polar. This ability to engage in hydrogen bonding leads to higher boiling points compared to non-polar compounds.
Boiling Points: Amines generally have higher boiling points than alkanes of similar molecular weight due to hydrogen bonding capabilities, particularly in primary and secondary amines. However, tertiary amines, which lack hydrogen atoms on the nitrogen, exhibit lower boiling points similar to those of ethers.
Odor: Many amines, especially low molecular weight ones, have a fishy odor. This property is notable in aliphatic amines used in certain agricultural products.
Chemical Properties
Basicity: Amines are basic due to the lone pair of electrons on the nitrogen atom, allowing them to accept protons (H⁺) from acids, forming ammonium salts. Their basic strength varies depending on whether the amine is primary, secondary, or tertiary, as well as on the nature of its substituents.
Reaction with Acids: Amines react with acids to form ammonium salts (e.g., R-NH₂ + HCl → R-NH₃⁺Cl⁻).
Structure and Properties of Amides
Amides are organic compounds containing the functional group -CONH₂, which consists of a carbonyl group (C=O) bonded to a nitrogen atom (N). They play a vital role in agriculture due to their use in fertilizers, pesticides, and soil conditioners, often impacting plant growth and soil properties. The general formula for an amide is R-CONH₂, R-CONHR', or R-CONR'R", where:
- R, R', and R" are either hydrogen atoms or organic substituents (alkyl or aryl groups).
- The presence of the carbonyl group (C=O) adjacent to the nitrogen creates a planar structure around the C-N bond.
- The bond between the carbonyl carbon and the nitrogen can exhibit partial double-bond character due to resonance, which limits rotation around this bond and increases stability.
:max_bytes(150000):strip_icc()/amide-56a12a103df78cf772680279.png) |
| General structure of an amide |
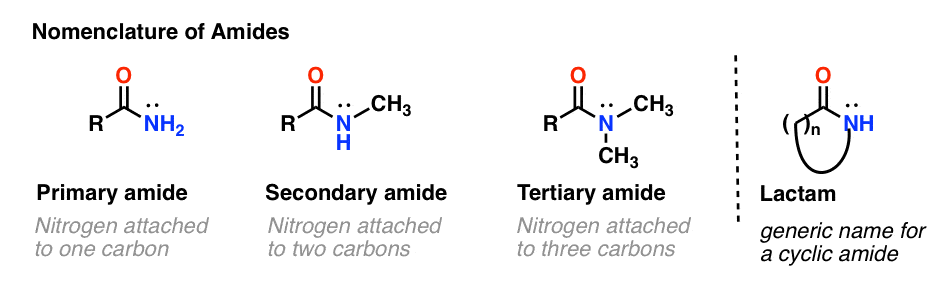
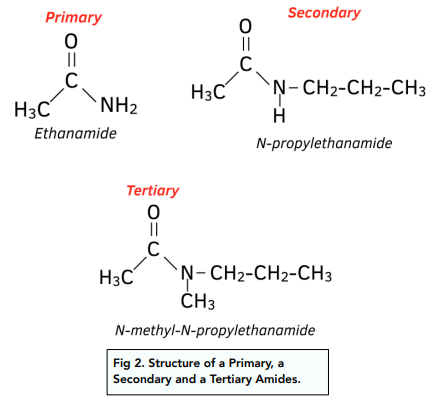
Classification of Amides
Amides are classified based on the number of substituents attached to the nitrogen:
- Primary amides (R-CONH₂): One carbonyl carbon is bonded to one nitrogen with no additional carbon groups attached to the nitrogen.
- Secondary amides (R-CONHR): The nitrogen is bonded to the carbonyl carbon and one additional carbon group.
- Tertiary amides (R-CONR₂): The nitrogen is bonded to the carbonyl carbon and two additional carbon groups.
Physical Properties
Polarity and Hydrogen Bonding: Amides are polar due to the carbonyl and NH groups, which can participate in hydrogen bonding. This hydrogen bonding increases the boiling and melting points of amides compared to other functional groups like esters or ketones.
Solubility: Low molecular weight amides (like formamide and acetamide) are generally soluble in water due to their ability to form hydrogen bonds. However, as the size of the R group increases, solubility in water decreases.
Melting and Boiling Points: Amides generally have high melting and boiling points because of strong intermolecular hydrogen bonds. Primary amides, in particular, have higher boiling points than secondary or tertiary amides.
State: Simple amides, such as formamide (liquid) and acetamide (solid), tend to exist in solid or liquid states at room temperature. Higher molecular weight amides are usually solid due to stronger intermolecular forces.
Chemical Properties
Acid and Base Stability: Amides are relatively stable under neutral and mild acidic or basic conditions, making them more resistant to hydrolysis than esters. However, under strong acidic or basic conditions, amides can be hydrolyzed.
Acidic Hydrolysis: Amides can be hydrolyzed in the presence of a strong acid to yield a carboxylic acid and ammonium ion (NH₄⁺).
Basic Hydrolysis: With a strong base, amides can be hydrolyzed to produce a carboxylate salt and ammonia (NH₃).
Reaction with Dehydrating Agents: Amides can be dehydrated to form nitriles using dehydrating agents such as phosphorus pentoxide (P₂O₅).
Formation of Amides (Amidation Reaction): Amides are typically synthesized by reacting a carboxylic acid derivative (like an acid chloride) with an amine or ammonia. This reaction is called amidation and is commonly used in organic synthesis (RCOOH + NH₃ → RCONH₂ +H₂O).
IUPAC Nomenclature of Amines
Naming Rules
Primary Amines:
IUPAC Nomenclature of Amides
Naming Rules
Problem Set
I. Provide the IUPAC names for the following compounds:
- CH₃NH₂
- CH₃CH₂NH₂
- CH₃CH₂CH₂NH₂
- CH₃CH₂CH₂CH₂NH₂
- CH₃CH₂CH₂CH₂CH₂NH₂
- CH₃NHCH₃
- CH₃CH₂NHCH₃
- CH₃CH₂NHCH₂CH₃
- C(CH₃)₂CHNHCH₃
- CH₃CH₂CH₂NHCH₃
- (CH₃)₃N
- CH₃CH₂N(CH₃)₂
- CH₃CH₂N(CH₂CH₃)₂
- CH₃(CH₂)₂N(CH₃)₂
- CH₃CH₂CH₂N(CH₂CH₃)₂
- CH₃CONH₂
- CH₃CH₂CONH₂
- CH₃(CH₂)₂CONH₂
- CH₃CON(CH₃)₂
- CH₃(CH₂)₄CONH₂
- CH₃CONHCH₃
- CH₃CH₂CONHCH₃
- CH₃(CH₂)₂CONHCH₃
- CH₃(CH₂)₃CONHCH₂CH₃
- CH₃(CH₂)₄CONHCH₃
- CH₃CON(CH₃)₂
- CH₃CON(CH₃)CH₂CH₃
- CH₃CH₂CON(CH₃)₂
- CH₃CON(CH₂CH₃)₂
- CH₃CH₂CON(CH₂CH₃)₂
II. Draw the line notation for the following compounds based on their IUPAC names:
- Hexan-1-amine
- Heptan-1-amine
- Octan-1-amine
- Nonan-1-amine
- 5-Methylhexan-1-amine
- N-Propylpentan-1-amine
- N-Propylhexan-1-amine
- N-Methylhexan-1-amine
- N-Methylheptan-1-amine
- N-Propylbutan-1-amine
- N,N-Dimethylpentanamine
- N,N-Dimethylhexanamine
- N,N-Diethylpentanamine
- N,N-Diethylhexanamine
- N-Ethyl-N-methylheptanamine
- Hexanamide
- Heptanamide
- Octanamide
- Nonanamide
- 5-Methylhexanamide
- N-Methylhexanamide
- N-Methylheptanamide
- N-Ethylpentanamide
- N-Ethylhexanamide
- N-Ethylheptanamide
- N,N-Dimethylpentanamide
- N,N-Dimethylhexanamide
- N,N-Diethylpentanamide
- N,N-Diethylhexanamide
- N-Ethyl-N-methylhexanamide
III. Complete the following reactions to form amides. Name the reactants and products.
- CH₃(CH₂)₂CH(CH₃)COOH + NH₃ →
- CH₃(CH₂)₃CH(CH₃)COOH + NH₃ →
- CH₃(CH₂)₇COOH + NH₃ →
- CH₃(CH₂)₆COOH + NH₃ →
- CH₃(CH₂)₅COOH + NH₃ →
- CH₃(CH₂)₄COOH + NH₃ →
- CH₃(CH₂)₃COOH + NH₃ →
- CH₃(CH₂)₂COOH + NH₃ →
- CH₃CH₂COOH + NH₃ →
- CH₃COOH + NH₃ →
.png)





.png)
.png)
.png)
Comments
Post a Comment