007 Aldehydes and Ketones
Aldehydes and Ketones
Aldehydes and ketones play significant roles in agriculture, contributing to both agrochemical production and preservation processes such as tanning and embalming. Aromatic aldehydes like benzaldehyde and cinnamaldehyde are responsible for the distinct fragrances and flavors in plants, with benzaldehyde being widely used in the perfumery industry. Additionally, aldehyde compounds serve as natural defense mechanisms in plants, protecting them from environmental stressors and herbivores. Ketones, particularly 2-ketones like 2-nonanone and 2-tridecanone, have been shown to stimulate plant growth and enhance antioxidant systems when delivered through solid lipid nanoparticles, as seen in crops like lettuce (Lactuca sativa). Moreover, aldehydes contribute to the characteristic aromas of plants; for instance, cis-3-hexenal imparts the fresh grassy scent of newly mowed lawns, while benzaldehyde and cinnamaldehyde are used as flavoring agents in food, providing almond and cinnamon notes respectively.
Learning Outcomes
Describe the structure and properties of aldehydes and ketones;
Apply IUPAC nomenclature to aldehydes and ketones; and
Understand and identify common reactions of aldehydes and ketones.
Aldehydes and ketones are crucial classes of organic compounds characterized by the presence of a carbonyl group (C=O). While both share this functional group, they differ in terms of structure, properties, and reactivity, which influence their distinct roles and applications in fields such as agriculture. Understanding these similarities and differences is key to appreciating their roles in agricultural practices.
Structural Differences and Similarities
- Aldehydes
- General Formula: Aldehydes follow the general formula R-CHO, where the R group can be a hydrogen atom or a hydrocarbon chain.
- Functional Group: The carbonyl group (C=O) in aldehydes is located at the end of the carbon chain, making aldehydes terminal functional groups.
- Ketones
- General Formula: Ketones have the general formula R-CO-R', where both R and R' are hydrocarbon groups.
- Functional Group: The carbonyl group in ketones is situated within the carbon chain, making ketones internal functional groups.
Despite both aldehydes and ketones containing the polar carbonyl group, the difference in positioning of this group affects their reactivity and properties. Aldehydes tend to be more reactive than ketones due to the presence of a hydrogen atom in aldehydes, which makes them more prone to oxidation.
Physical and Chemical Properties
Polarity
- Both aldehydes and ketones are polar molecules because of the electronegativity difference between the oxygen and carbon in the carbonyl group. This polarity influences their solubility and reactivity.
Solubility
- Aldehydes: Smaller aldehydes (e.g., formaldehyde, acetaldehyde) are soluble in water due to their polarity, but larger aldehydes with longer carbon chains become less soluble in water as the nonpolar hydrocarbon part dominates.
- Ketones: Ketones exhibit similar solubility patterns, with smaller ketones like acetone being soluble in water. Larger ketones are more soluble in organic solvents due to their hydrophobic hydrocarbon groups.
Odor
- Aldehydes tend to have strong, often pleasant odors, which are sometimes used in agricultural products or food flavorings. For example, benzaldehyde gives an almond-like aroma and can be found in certain flavoring agents.
- Ketones also exhibit distinct odors, which make them useful in fragrances. Acetone, for instance, is commonly used as a solvent but has a sharp smell, whereas other ketones like cyclohexanone are used industrially for their solvent properties.
Physical State
- Aldehydes: Smaller aldehydes, such as methanal (formaldehyde), are gases at room temperature, whereas larger aldehydes are typically liquids or solids.
- Ketones: Most common ketones are liquids at room temperature, with acetone being a notable example.
- The physical state of these compounds influences their handling and storage in agricultural settings. For example, formaldehyde’s gaseous state allows for its use as a fumigant or preservative.
Chemical Properties
Reactivity
- Aldehydes: Aldehydes are more reactive than ketones due to the presence of a hydrogen atom attached to the carbonyl carbon. This hydrogen makes aldehydes more susceptible to nucleophilic attack, and they are easily oxidized to carboxylic acids.
- Ketones: Ketones are less reactive because they have two hydrocarbon groups attached to the carbonyl carbon, creating steric hindrance and stabilizing the molecule. This makes ketones less electrophilic and more resistant to oxidation.
In agriculture, this difference in reactivity can be critical when choosing aldehydes for processes requiring fast reactions, such as the synthesis of certain herbicides. Ketones, being more stable, might be preferable in formulations requiring longevity.
Common Reactions
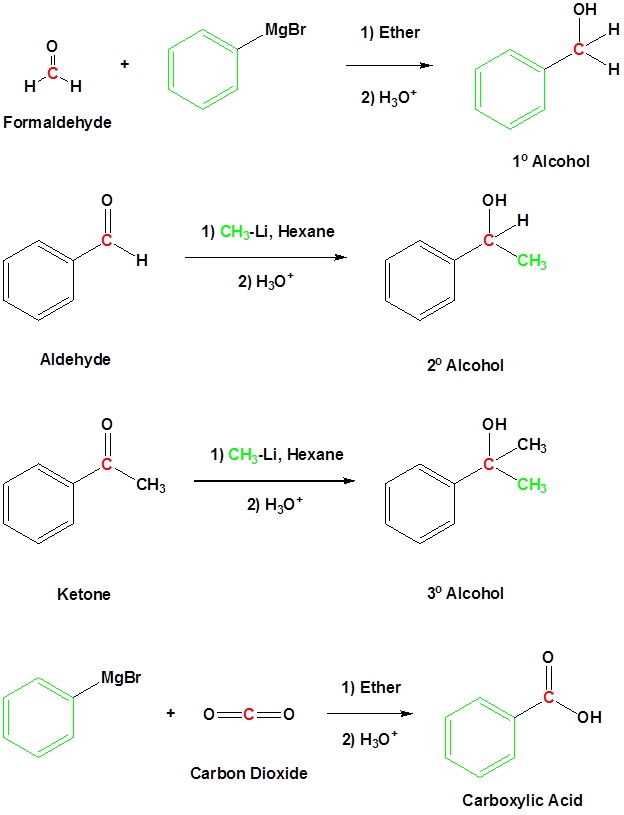
Nucleophilic Addition: Both aldehydes and ketones undergo nucleophilic addition due to the electrophilic nature of the carbonyl carbon. This reaction is essential in synthesizing various agricultural chemicals, including fertilizers and pesticides. When reacting with Grignard reagents (RMgX), aldehydes and ketones form alcohols after hydrolysis:
- Aldehyde: RCHO+RMgX→RCH(OH)R
- Ketone: RCO+RMgX→RC(OH)R
Hydride Reduction: Aldehydes and ketones can be reduced to alcohols using reducing agents like sodium borohydride (NaBH₄) or lithium aluminum hydride (LiAlH₄). Aldehydes are reduced to primary alcohols, while ketones are reduced to secondary alcohols:
- Aldehyde: RCHO+NaBH4→RCH2OH
- Ketone: RCO+NaBH4→RCHOH
This reaction is used in agriculture for synthesizing alcohol-based compounds, which are vital for creating certain agrochemicals.
Oxidation:
- Aldehydes: Aldehydes are easily oxidized to carboxylic acids using mild oxidizing agents like Tollens' reagent or dichromate: RCHO+[O]→RCOOH
- Ketones: Ketones are generally resistant to oxidation and require strong oxidizing agents to cleave the carbon chain or convert them into carboxylic acids.
Oxidation reactions are important in agriculture, especially in the creation of acids used in fertilizers or soil treatments. The easier oxidation of aldehydes can be utilized in processes that require rapid chemical transformations.
Reactivity
- Aldehydes: Aldehydes are more reactive than ketones due to the presence of a hydrogen atom attached to the carbonyl carbon. This hydrogen makes aldehydes more susceptible to nucleophilic attack, and they are easily oxidized to carboxylic acids.
- Ketones: Ketones are less reactive because they have two hydrocarbon groups attached to the carbonyl carbon, creating steric hindrance and stabilizing the molecule. This makes ketones less electrophilic and more resistant to oxidation.
In agriculture, this difference in reactivity can be critical when choosing aldehydes for processes requiring fast reactions, such as the synthesis of certain herbicides. Ketones, being more stable, might be preferable in formulations requiring longevity.
Common Reactions
Nucleophilic Addition: Both aldehydes and ketones undergo nucleophilic addition due to the electrophilic nature of the carbonyl carbon. This reaction is essential in synthesizing various agricultural chemicals, including fertilizers and pesticides. When reacting with Grignard reagents (RMgX), aldehydes and ketones form alcohols after hydrolysis:
- Aldehyde: RCHO+RMgX→RCH(OH)R
- Ketone: RCO+RMgX→RC(OH)R
Hydride Reduction: Aldehydes and ketones can be reduced to alcohols using reducing agents like sodium borohydride (NaBH₄) or lithium aluminum hydride (LiAlH₄). Aldehydes are reduced to primary alcohols, while ketones are reduced to secondary alcohols:
- Aldehyde: RCHO+NaBH4→RCH2OH
- Ketone: RCO+NaBH4→RCHOH
This reaction is used in agriculture for synthesizing alcohol-based compounds, which are vital for creating certain agrochemicals.
Oxidation:
- Aldehydes: Aldehydes are easily oxidized to carboxylic acids using mild oxidizing agents like Tollens' reagent or dichromate: RCHO+[O]→RCOOH
- Ketones: Ketones are generally resistant to oxidation and require strong oxidizing agents to cleave the carbon chain or convert them into carboxylic acids.
Oxidation reactions are important in agriculture, especially in the creation of acids used in fertilizers or soil treatments. The easier oxidation of aldehydes can be utilized in processes that require rapid chemical transformations.
How to Name Aldehydes and Ketones
Aldehydes and ketones are named according to the IUPAC (International Union of Pure and Applied Chemistry) nomenclature system, which provides systematic rules for naming organic compounds. Here’s a detailed overview of how each class of compounds is named:
Aldehydes and ketones are named according to the IUPAC (International Union of Pure and Applied Chemistry) nomenclature system, which provides systematic rules for naming organic compounds. Here’s a detailed overview of how each class of compounds is named:
Naming Aldehydes
- Identify the Longest Carbon Chain: The longest carbon chain containing the carbonyl group (C=O) is identified. The carbonyl group must be at the end of the chain.
- Number the Carbon Chain: Number the carbon chain starting from the end nearest to the carbonyl group. This ensures that the carbonyl carbon receives the lowest possible number (1).
- Replace the Suffix with "-al": Replace the "-e" ending of the corresponding alkane name with "-al" to indicate the presence of the aldehyde functional group.
Examples
- Formaldehyde: The simplest aldehyde, with one carbon atom (methanal).
- Acetaldehyde: The aldehyde with two carbon atoms (ethanal).
- Butyraldehyde: The aldehyde with four carbon atoms (butanal).
Summary of Aldehyde Naming
- General formula: R-CHO
- Example: Pentanal (C5H10O) is named from pentane by replacing "-e" with "-al."
Naming Ketones
- Identify the Longest Carbon Chain: The longest carbon chain containing the carbonyl group is identified. The carbonyl group must be within the chain.
- Number the Carbon Chain: Number the carbon chain starting from the end nearest to the carbonyl group to give the carbonyl carbon the lowest possible number.
- Replace the Suffix with "-one": Replace the "-e" ending of the corresponding alkane name with "-one" to indicate the presence of the ketone functional group.
- Indicate the Position: The position of the carbonyl group is indicated by a number before the suffix "-one."
Examples
- Acetone: The simplest ketone with three carbon atoms (propan-2-one).
- Butanone: A ketone with four carbon atoms (butan-2-one).
- Cyclohexanone: A cyclic ketone with six carbon atoms (cyclohexan-2-one).
Summary of Ketone Naming
- General formula: R-CO-R'
- Example: 3-Pentanone (C5H10O) is named from pentane by replacing "-e" with "-one" and indicating the position of the carbonyl group.
Summary of Naming Rules
- Aldehydes: Named by replacing "-e" with "-al" and numbering the carbon chain from the carbonyl end.
- Ketones: Named by replacing "-e" with "-one," indicating the position of the carbonyl group.
By following these systematic rules, aldehydes and ketones can be accurately named, allowing for clear communication in organic chemistry.
Relevance in Agriculture
Aldehydes in Agriculture
- Aldehydes, such as formaldehyde, are used as preservatives and disinfectants in agriculture. They help in extending the shelf life of seeds and plants by preventing microbial growth.
- Aldehydes also play a role in the flavor and scent profiles of agricultural products. For instance, acetaldehyde naturally occurs in ripe fruits and influences their aroma, which is critical for both fresh produce markets and the food processing industry.
Ketones in Agriculture
- Ketones like acetone are widely used as solvents in pesticide formulations and other agrochemicals. Their solvent properties allow for the efficient application of active ingredients in agricultural treatments.
- In some plants, ketones are involved in metabolic processes and the production of secondary metabolites, which can influence plant defense mechanisms or contribute to plant growth.
Problem Set
I. Provide the IUPAC names for the following compounds:
- CH₃CH(CH₃)CH₂CH₂CHO
- CH₃C(=O)CH(CH₃)CH₂CH₃
- CH₃(CH₂)₂C(=O)CH(CH₃)₂
- CH₃C(=O)CH₂CH(CH₃)CH₂CH₃
- CH₃(CH₂)₃C(=O)C(CH₃)₂
- CH₃CH(CH₃)C(=O)CH₂CH₃
- CH₃C(=O)CH(CH₃)CH₂CH₃
- CH₃C(CH₃)₂C(=O)CH₂CH₃
- CH₃(CH₂)₄C(=O)C(CH₃)₂
- CH₃C(=O)CH(CH₃)CH₂CH₂CH₃
- CH₃(CH₂)₃C(=O)C(CH₃)₃
- CH₃C(=O)C(CH₃)CH₂CH₃
- CH₃C(CH₃)₂C(=O)CH₂(CH₃)
- CH₃CH(CH₃)C(=O)C(CH₃)₂
- CH₃C(=O)C(CH₃)CH₂CH₂CH₃
II. Draw the line notation for the following compounds based on their IUPAC names:
- 3-Methylpentanal
- 4-Methylhexan-2-one
- 3-Methylhexan-2-one
- 2,3-Dimethylpentan-2-one
- 4-Ethylheptan-3-one
- 2,2,4-Trimethylpentan-3-one
- 3,4-Dimethylheptan-2-one
- 5,5-Dimethylhexan-2-one
- 2-Ethyl-3,3-dimethylhexan-4-one
- 4,4-Diethylheptan-3-one
- 2,2,5,5-Tetramethylhexan-3-one
- 3-Ethyl-2,2-dimethylpentan-3-one
- 4,4-Diethyl-3-methylheptan-2-one
- 2,2,3,3-Tetramethylpentan-4-one
- 3,3,5,5-Tetramethylheptan-4-one
III. Predict the product alcohol (IUPAC name and structure) given the aldehyde/ketone and Grignard reagent. Writing the complete chemical reaction. Identifying the type of alcohol formed.
- Aldehyde: 3-Methylpentanal; Grignard Reagent: CH₃MgBr
- Ketone: 4-Methylhexan-2-one; Grignard Reagent: CH₃MgBr
- Aldehyde: 2-Ethylhexanal; Grignard Reagent: C₂H₅MgBr
- Ketone: 3,3-Dimethylpentan-2-one; Grignard Reagent: CH₃MgBr
- Aldehyde: 4-Ethylpentanal; Grignard Reagent: CH₃MgBr
- Ketone: 2-Pentanone; Grignard Reagent: CH₃MgBr
- Aldehyde: 3-Methylbutanal; Grignard Reagent: C₃H₇MgBr
- Ketone: 2-Hexanone; Grignard Reagent: CH₃MgBr
- Aldehyde: 2,4-Dimethylpentanal; Grignard Reagent: CH₃MgBr
- Ketone: 3-Ethyl-2-butanone; Grignard Reagent: CH₃MgBr
- Aldehyde: 3-Phenylbutanal; Grignard Reagent: C₆H₅MgBr
- Ketone: 4-Methyl-3-heptanone; Grignard Reagent: CH₃MgBr
- Aldehyde: 2-Phenylpentanal; Grignard Reagent: C₆H₅MgBr
- Ketone: 2,3-Dimethyl-3-hexanone; Grignard Reagent: CH₃MgBr
- Aldehyde: 4-Methyl-3-phenylbutanal; Grignard Reagent: C₆H₅MgBr
IV. Identify the starting aldehyde/ketone and Grignard reagent given the alcohol product. Write the balanced reaction.
- 3-Methyl-2-hexanol
- Alcohol Product: 4-Methyl-3-heptanol
- Alcohol Product: 2-Ethyl-3-heptanol
- Alcohol Product: 3,3-Dimethyl-2-hexanol
- Alcohol Product: 4-Ethyl-2-hexanol
- Alcohol Product: 3-Pentanol
- Alcohol Product: 3-Methyl-2-hexanol
- Alcohol Product: 3-Hexanol
- Alcohol Product: 2,4-Dimethyl-3-hexanol
- Alcohol Product: 3-Ethyl-3-pentanol
- Alcohol Product: 3-Phenyl-2-pentanol
- Alcohol Product: 4-Methyl-3-octanol
- Alcohol Product: 2-Phenyl-3-pentanol
- Alcohol Product: 2,3-Dimethyl-3-heptanol
- Alcohol Product: 4-Methyl-3-phenyl-2-pentanol
.png)



.png)
.png)
.png)
Comments
Post a Comment