008 Carboxylic Acids and Esters
Learning Outcomes
Describe the structure and properties of carboxylic acids and esters;
Apply IUPAC nomenclature to carboxylic acids, and esters; and
Understand and identify common reactions of carboxylic acids and esters.
Carboxylic Acids and Esters in Daily Life and Agriculture
Carboxylic acids and esters are essential in numerous industries due to their unique properties and versatility. Carboxylic acids are widely used in food, personal care, pharmaceuticals, household products, and industrial applications. Esters, known for their pleasant odors, are key in flavorings, fragrances, solvents, plasticizers, biodegradable products, and cleaning supplies. Both compounds play a crucial role in the consumer products we encounter daily.
Esters and carboxylic acids are essential in agriculture, serving diverse functions. Esters are used as active ingredients in pesticides, herbicides, and plant growth regulators, as well as solvents, flavoring agents, and biodegradable plastics. While they enhance agricultural practices, certain esters can pose environmental risks through contamination. Carboxylic acids improve soil health, enhance nutrient availability, act as plant growth regulators, and aid in fertilization, making nutrients more accessible to plants. Both compounds contribute to higher crop yields and quality, supporting sustainable farming methods.
Structure and Properties of Carboxylic Acids
Carboxylic acids are organic compounds characterized by the presence of a carboxyl group (−COOH), which consists of a carbonyl group (C=O) and a hydroxyl group (−OH) attached to the same carbon atom. The general formula for carboxylic acids can be represented as RCOOH, where R is a hydrocarbon chain that can vary in length and structure.
Structural Characteristics
Hybridization and Geometry: The carbon atom in the carboxyl group is sp² hybridized, resulting in a trigonal planar geometry with bond angles of approximately 120°. This structure allows for resonance stabilization, where the lone pair of electrons on the hydroxyl oxygen can delocalize into the carbonyl π system, enhancing the stability of the molecule.
Dimer Formation: Carboxylic acids often exist as cyclic dimers due to strong hydrogen bonding between molecules. Each acid can form two hydrogen bonds with another molecule, leading to higher boiling points compared to alcohols, which can only form one hydrogen bond per molecule.
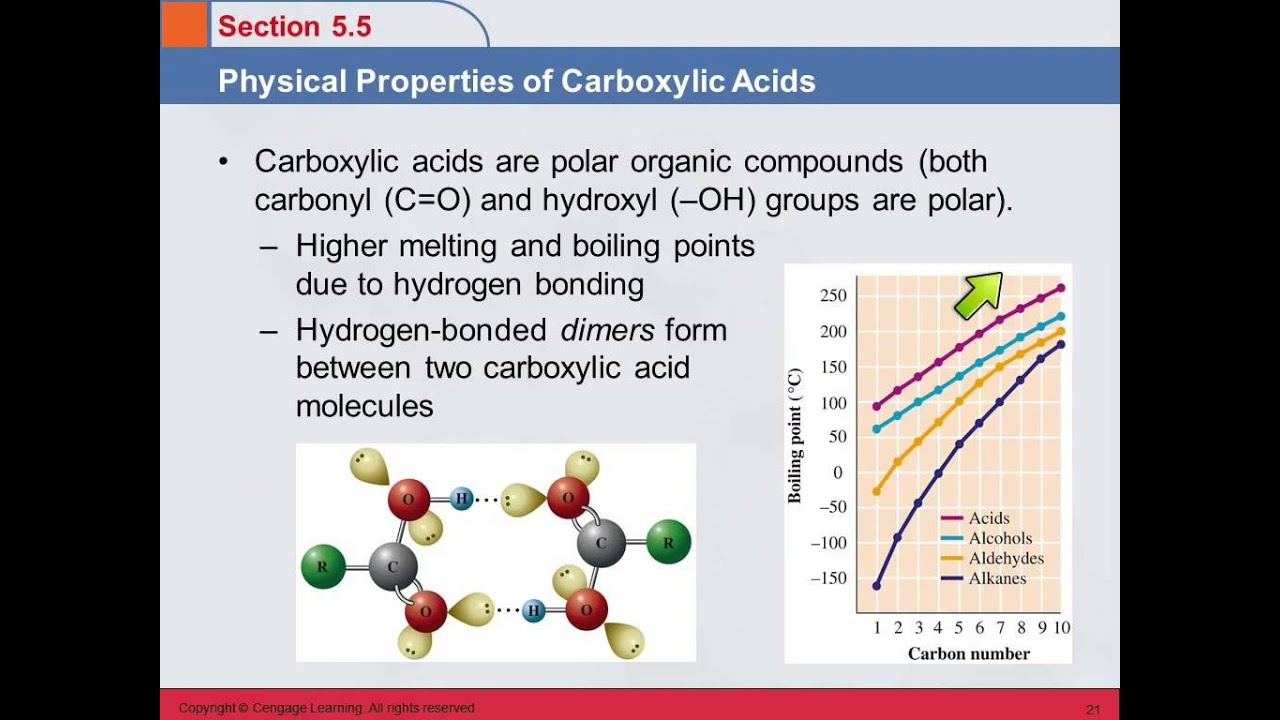
Physical Properties
Polarity and Solubility: Carboxylic acids are polar compounds, which contributes to their solubility in water, particularly for smaller acids. However, as the carbon chain length increases (typically beyond six carbons), their solubility decreases significantly.
Boiling Points: The boiling points of carboxylic acids are generally higher than those of corresponding alcohols due to their ability to form dimers through hydrogen bonding. For example, acetic acid has a boiling point of 117.9 °C, while ethanol boils at 78.3 °C despite both having similar molecular weights.
Chemical Properties
Acidity: Carboxylic acids are classified as weak acids compared to mineral acids like hydrochloric acid but are stronger than alcohols and phenols. They dissociate in aqueous solutions to produce carboxylate ions (RCOO- ) and hydronium ions (H3O+). The acidity constant (Ka) for common carboxylic acids typically ranges from 10-4 to 10-5. For instance, acetic acid has a Ka value of 1.75 × 10-5, indicating that only about 0.1% of acetic acid molecules dissociate in solution.
Resonance Stabilization: The greater acidity of carboxylic acids compared to alcohols is attributed to the resonance stabilization of their conjugate base (carboxylate ion), where the negative charge is delocalized over two oxygen atoms, making it more stable than the alkoxide ion formed from alcohols.
Structure and Properties of Esters
Esters are derived from carboxylic acids by replacing the hydroxyl group (−OH) with an alkoxy group (−O-R). The general formula for esters can be expressed as RCOOR', where R is the hydrocarbon chain from the carboxylic acid, and R' is from an alcohol.
Structural Characteristics
Geometry: Like carboxylic acids, esters also have a trigonal planar configuration around the carbonyl carbon due to sp² hybridization. The bond angles are similarly around 120°.
Lack of Hydrogen Bonding: Esters do not form hydrogen bonds with themselves like carboxylic acids do; instead, they exhibit weaker dipole-dipole interactions. This results in lower boiling points compared to their corresponding carboxylic acids.
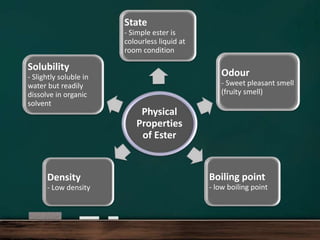
Physical Properties
Solubility: Esters are generally less polar than carboxylic acids and may have lower solubility in water, particularly as their hydrocarbon chains become longer. Small esters, however, can still dissolve in water due to their ability to engage in hydrogen bonding with water molecules.
Odor and Flavor: Many esters are known for their pleasant fruity odors and flavors, which makes them widely used in food flavorings and fragrances.
Chemical Properties
Reactivity: Esters can undergo hydrolysis (reaction with water) to revert back into carboxylic acids and alcohols under acidic or basic conditions. This reaction is important in both synthetic organic chemistry and biological processes such as digestion.
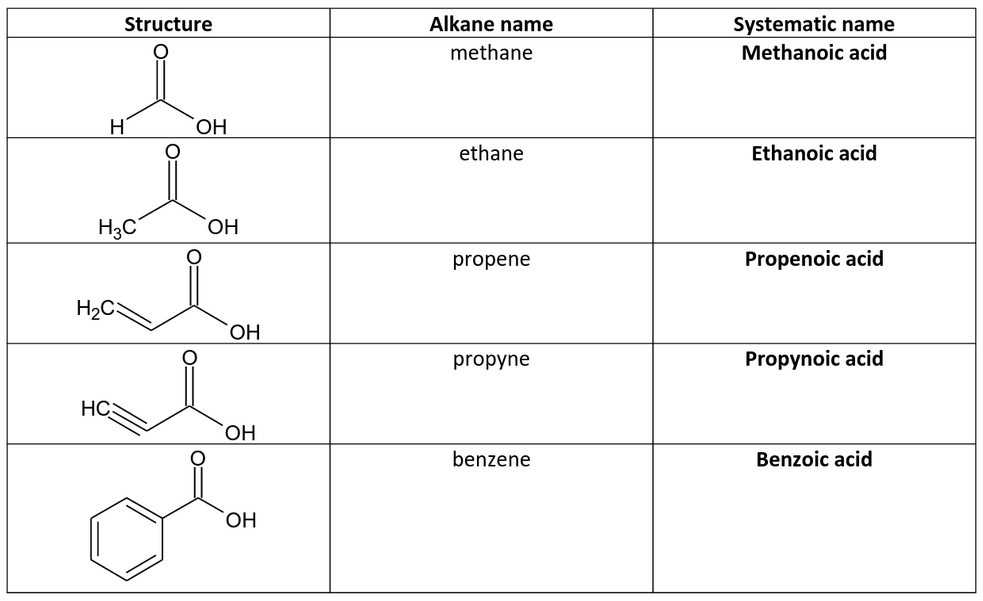
IUPAC Nomenclature of Carboxylic Acids
Naming Rules
- Identify the Longest Carbon Chain: Locate the longest continuous carbon chain that contains the carboxyl group (-COOH). This chain determines the base name of the acid.
- Suffix Addition: The name of the compound is derived by adding the suffix **-oic acid** to the root name of the alkane corresponding to the longest chain. For example, a three-carbon chain (propane) becomes **propanoic acid**.
- Numbering: Number the carbon chain starting from the end nearest to the carboxyl group, which is always assigned as carbon 1. Substituents are named and numbered accordingly.
Examples
- Acetic Acid: Derived from ethane, it is named ethanoic acid (C2H4O2).
- Butyric Acid: Derived from butane, it is named butanoic acid (C4H8O2).
- Benzoic Acid: A carboxylic acid derived from benzene, it retains its name as benzoic acid.
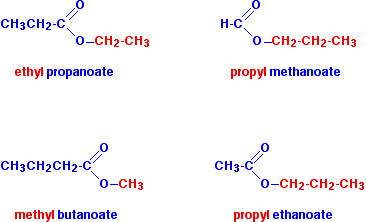
IUPAC Nomenclature of Esters
Naming Rules
Identify Alkyl Group: The alkyl group from the alcohol is named first. This group is derived from the alcohol used in the esterification process and ends with −yl.
- Identify Parent Chain: The part of the ester containing the carbonyl group (−C(=O)−) is derived from the carboxylic acid. The name of this portion ends with −oate, replacing the suffix -oic acid.
- No Numbering for Alkyl Group: No locant number is assigned to the alkyl group; it simply precedes the name of the acid-derived portion.
Examples
- Ethyl Acetate: Formed from acetic acid and ethanol, it can also be referred to as ethyl ethanoate (C4H8O2).
- Methyl Butanoate: Derived from butanoic acid and methanol (C5H10O2).
- Propyl Propanoate: Formed from propanoic acid and propanol (C5H10O2).
Common Reactions of Carboxylic Acids
Carboxylic acids and esters are important functional groups in organic chemistry, each participating in a variety of reactions. Below is an overview of common reactions involving these compounds.
- Formation of Esters (Fischer Esterification): Carboxylic acids react with alcohols in the presence of an acid catalyst to form esters and water.
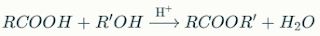
General reaction of Fisher esterification - Acid-Base Reactions: Carboxylic acids can react with strong bases to form carboxylate salts.
General reaction of acid-base reactions of carboxylic acids - Conversion to Acid Chlorides: Carboxylic acids react with thionyl chloride (SOCl₂) or phosphorus trichloride (PCl₃) to form acyl chlorides. This reaction enhances the reactivity of the carboxylic acid for further transformations.
General reaction of conversion to acid chlorides using phosphorus trichloride General reaction of conversion to acid chlorides using thionyl chloride - Formation of Amides: - Direct conversion of carboxylic acids to amides can be challenging due to the formation of unreactive carboxylate ions. However, using coupling agents like Dicyclohexylcarbodiimide (DCC) can facilitate this reaction.
General reaction of formation of amides using DCC - Decarboxylation: Carboxylic acids can undergo decarboxylation, losing carbon dioxide when heated with soda lime or other reagents.
General reaction of decarboxylation - Reduction to Alcohols: Carboxylic acids can be reduced to primary alcohols using reducing agents like lithium aluminum hydride (LiAlH₄).
General reaction of reduction to alcohol
Common Reactions of Esters
- Hydrolysis: Esters can be hydrolyzed in the presence of water and an acid or base to yield carboxylic acids and alcohols.
General base-catalyzed reaction General acid-catalyzed reaction - Transesterification: Esters can react with alcohols to form new esters and different alcohols.
General reaction of transesterification - Reduction to Alcohols: Esters can be reduced to primary alcohols using lithium aluminum hydride (LiAlH₄).
General reaction of ester's reduction to alcohols - Formation of Anhydrides: Esters can react with carboxylic acids in the presence of a base to form anhydrides.
General reaction of the formation of anhydrides
Both carboxylic acids and esters participate in a variety of chemical reactions that are fundamental to organic synthesis, including esterification, hydrolysis, reduction, and nucleophilic substitutions, each providing pathways for the transformation and utilization of these functional groups in various applications.
Problem Set
I. Provide the IUPAC names for the following compounds:
- CH₃CH₂CH₂COOH
- CH₃(CH₂)₄COOH
- CH₃CH(CH₃)CH₂COOH
- CH₃(CH₂)₅COOH
- CH₃CH₂CH₂CH₂CH₂COOH
- CH₃CH₂CH(CH₃)COOH
- CH₃(CH₂)₆COOH
- CH₃CH₂CH₂C(=O)OCH₃
- CH₃(CH₂)₂C(=O)OCH₂CH₃
- CH₃CH₂C(=O)OCH₂CH₃
- CH₃CH₂CH₂C(=O)OCH₂CH₂CH₃
- CH₃CH₂CH₂CH₂C(=O)OCH₃
- CH₃(CH₂)₄C(=O)OCH₂CH₃
- CH₃CH₂CH₂C(=O)OCH₂CH₂CH₃
- CH₃C(=O)OCH₂CH₂CH₂CH₃
II. Draw the line notation for the following compounds based on their IUPAC names:
- Propyl propanoate
- Methyl hexanoate
- Isopropyl butanoate
- Butyl acetate
- Methyl heptanoate
- Ethyl hexanoate
- Pentyl butanoate
- Pentanoic acid
- 2-Methylbutanoic acid
- Hexanoic acid
- 3-Methylpentanoic acid
- Heptanoic acid
- 2,2-Dimethylpropanoic acid
- 4-Methylpentanoic acid
- Octanoic acid
III. For each given carboxylic acid, write the balanced chemical reactions for:
a.) Ester formation
b.) Acid-base reaction
c.) Conversion to acid chlorides
d.) Amide formation
e.) Decarboxylation
f.) Reduction to alcohol
- Pentanoic acid
- 2-Methylbutanoic acid
- Hexanoic acid
- 3-Methylpentanoic acid
- Heptanoic acid
- 2,2-Dimethylpropanoic acid
- 4-Methylpentanoic acid
- Octanoic acid
IV. For each given ester, write the balanced chemical reactions for:
a.) Hydrolysis
b.) Transesterification
c.) Reduction to alcohols
d.) Formation of Anhydrides
- Propyl propanoate
- Methyl hexanoate
- Isopropyl butanoate
- Butyl acetate
- Methyl heptanoate
- Ethyl hexanoate
- Pentyl butanoate
.png)





.png)
.png)
.png)
Comments
Post a Comment