005 Alcohols
Alcohols
Alcohols, particularly in the form of ethanol, are relevant to agriculture through their use as biofuels, their influence on crop production and livestock feeding, and the safety implications for pesticide handling. Additionally, the environmental impact of alcohol production raises concerns about resource allocation and food security. As agricultural practices evolve, understanding these dynamics is crucial for promoting sustainable farming and ensuring the safety of agricultural workers.
Alcohols are a significant class of organic compounds characterized by the presence of one or more hydroxyl (-OH) functional groups attached to carbon atoms.
Structure of Alcohols
Alcohols can be represented by the general formula R-OH, where R is an alkyl group. The hydroxyl group (-OH) is the defining feature of alcohols, and its position on the carbon chain determines the classification of the alcohol as primary, secondary, or tertiary:
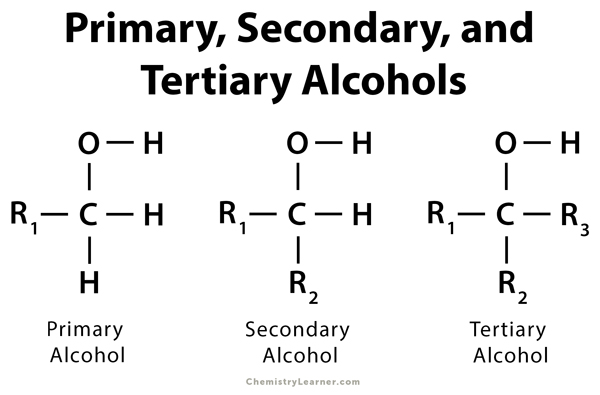
- Primary Alcohols (1°): The carbon atom bonded to the -OH group is attached to only one other carbon atom (e.g., ethanol, CH3CH2OH).
- Secondary Alcohols (2°): The carbon atom with the -OH group is connected to two other carbon atoms (e.g., isopropanol, (CH3)2CHOH).
- Tertiary Alcohols (3°): The carbon atom with the -OH group is attached to three other carbon atoms (e.g., tert-butanol, (CH3)3COH).
Physical Characteristics
Alcohols exhibit several notable physical properties:
- Polarity and Solubility: The hydroxyl group is polar, allowing alcohols to form hydrogen bonds with water, which enhances their solubility in water, especially for lower molecular weight alcohols. As the carbon chain length increases, solubility decreases due to the hydrophobic character of the alkyl group[2][4].
- Boiling Points: Alcohols generally have higher boiling points than their corresponding alkanes due to the presence of hydrogen bonding. For example, ethanol has a significantly higher boiling point than ethane, reflecting the stronger intermolecular forces in alcohols.
- Physical State: Most alcohols are colorless liquids or solids at room temperature, with low molecular weight alcohols being highly volatile and flammable.
Chemical Characteristics
Alcohols can undergo various chemical reactions, including:
- Dehydration: Alcohols can lose water to form alkenes, especially when heated in the presence of an acid catalyst.
- Oxidation: Primary alcohols can be oxidized to aldehydes and then to carboxylic acids, while secondary alcohols are oxidized to ketones. Tertiary alcohols do not oxidize easily.
- Esterification: Alcohols react with acids to form esters, a reaction commonly used in the production of fragrances and flavorings.
Common Reactions
- Oxidation Reactions: Oxidation reactions of alcohols are fundamental transformations in organic chemistry, involving the conversion of alcohols into more oxidized compounds such as aldehydes, ketones, and carboxylic acids (primary alcohols → aldehydes → carboxylic acids; secondary alcohols → ketones; tertiary alcohols → no reaction under mild conditions)
- Dehydration: Alcohols can be converted to alkenes by removing water, often using heat and an acid catalyst.
- Ester Formation: Alcohols react with carboxylic acids to form esters and water.
Isomerism
Alcohols can exhibit structural isomerism based on the arrangement of carbon atoms and the position of the hydroxyl group. Isomerism can be classified as:
- Chain Isomerism: Different arrangements of the carbon skeleton (e.g., butanol can exist as n-butanol, isobutanol).
- Position Isomerism: Variations in the position of the hydroxyl group on the carbon chain (e.g., 1-butanol vs. 2-butanol).
Alcohols are versatile compounds with distinct structural features, physical and chemical properties, and various applications in industry and daily life. Their reactivity and isomerism contribute to their importance in organic chemistry and agriculture.
Alcohols
Alcohols, particularly in the form of ethanol, are relevant to agriculture through their use as biofuels, their influence on crop production and livestock feeding, and the safety implications for pesticide handling. Additionally, the environmental impact of alcohol production raises concerns about resource allocation and food security. As agricultural practices evolve, understanding these dynamics is crucial for promoting sustainable farming and ensuring the safety of agricultural workers.
Alcohols are a significant class of organic compounds characterized by the presence of one or more hydroxyl (-OH) functional groups attached to carbon atoms. Their structure, physical and chemical properties, common reactions, and isomerism can be summarized as follows:
Structure of Alcohols
Alcohols can be represented by the general formula R-OH, where R is an alkyl group. The hydroxyl group (-OH) is the defining feature of alcohols, and its position on the carbon chain determines the classification of the alcohol as primary, secondary, or tertiary:
- Primary Alcohols (1°): The carbon atom bonded to the -OH group is attached to only one other carbon atom (e.g., ethanol, CH3CH2OH).
- Secondary Alcohols (2°): The carbon atom with the -OH group is connected to two other carbon atoms (e.g., isopropanol, (CH3)2CHOH).
- Tertiary Alcohols (3°): The carbon atom with the -OH group is attached to three other carbon atoms (e.g., tert-butanol, (CH3)3COH).
Physical Characteristics
Alcohols exhibit several notable physical properties:
- Polarity and Solubility: The hydroxyl group is polar, allowing alcohols to form hydrogen bonds with water, which enhances their solubility in water, especially for lower molecular weight alcohols. As the carbon chain length increases, solubility decreases due to the hydrophobic character of the alkyl group[2][4].
- Boiling Points: Alcohols generally have higher boiling points than their corresponding alkanes due to the presence of hydrogen bonding. For example, ethanol has a significantly higher boiling point than ethane, reflecting the stronger intermolecular forces in alcohols.
- Physical State: Most alcohols are colorless liquids or solids at room temperature, with low molecular weight alcohols being highly volatile and flammable.
Chemical Characteristics
Alcohols can undergo various chemical reactions, including:
- Dehydration: Alcohols can lose water to form alkenes, especially when heated in the presence of an acid catalyst.
- Oxidation: Primary alcohols can be oxidized to aldehydes and then to carboxylic acids, while secondary alcohols are oxidized to ketones. Tertiary alcohols do not oxidize easily.
- Esterification: Alcohols react with acids to form esters, a reaction commonly used in the production of fragrances and flavorings.
Common Reactions
- Oxidation Reactions: Oxidation reactions of alcohols are fundamental transformations in organic chemistry, involving the conversion of alcohols into more oxidized compounds such as aldehydes, ketones, and carboxylic acids (primary alcohols → aldehydes → carboxylic acids; secondary alcohols → ketones; tertiary alcohols → no reaction under mild conditions)
- Dehydration: Alcohols can be converted to alkenes by removing water, often using heat and an acid catalyst.
- Ester Formation: Alcohols react with carboxylic acids to form esters and water.
Isomerism
Alcohols can exhibit structural isomerism based on the arrangement of carbon atoms and the position of the hydroxyl group. Isomerism can be classified as:
- Chain Isomerism: Different arrangements of the carbon skeleton (e.g., butanol can exist as n-butanol, isobutanol).
- Position Isomerism: Variations in the position of the hydroxyl group on the carbon chain (e.g., 1-butanol vs. 2-butanol).
Alcohols are versatile compounds with distinct structural features, physical and chemical properties, and various applications in industry and daily life. Their reactivity and isomerism contribute to their importance in organic chemistry and agriculture.
Basic Principles of Naming Alcohols
Naming alcohols involves following specific rules established by the International Union of Pure and Applied Chemistry (IUPAC). Here’s a comprehensive guide to the nomenclature of alcohols:
- Identify the Parent Chain, Longest Carbon Chain: Determine the longest continuous chain of carbon atoms that contains the hydroxyl (-OH) group. This chain will serve as the parent compound.
- Change the Suffix, Replace the -e with -ol: The name of the parent alkane is modified by removing the -e ending and adding -ol to indicate the presence of the hydroxyl group. For example: methane (C₁) becomes methanol (C₁H₃OH), ethane (C₂) becomes ethanol (C₂H₅OH).
- Number the Parent Chain, Lowest Locant for -OH: Number the carbon atoms in the chain starting from the end closest to the hydroxyl group. This ensures that the -OH group receives the lowest possible number. For instance, in 1-propanol (C₃H₇OH), the -OH group is on the first carbon, and in 2-propanol, the -OH group is on the second carbon.
- Indicate Substituents, Number and Name Substituents: If there are other substituent groups (alkyl groups, halogens, etc.) on the carbon chain, they should be named and numbered according to their position on the chain. List them in alphabetical order in the final name. For example, in 3-methyl-2-butanol, the substituent (methyl) is indicated along with its position.
Examples of Alcohol Naming
- Methanol: CH₃OH (from methane)
- Ethanol: CH₃CH₂OH (from ethane)
- 1-Propanol: CH₃CH₂CH₂OH (from propane)
- 2-Propanol**: (CH₃)₂CHOH (from propane)
- Cyclohexanol**: C₆H₁₁OH (cyclic structure with -OH at C1)
By following these guidelines, one can accurately name a wide variety of alcohols according to IUPAC standards.


.png)
.png)
.png)
Comments
Post a Comment