004 Alkynes
Alkynes
Alkynes are currently relevant to agriculture through their use in agrochemical synthesis and as markers for studying diet composition in livestock. With ongoing research, their potential applications may expand further in the future.
Alkynes are a class of unsaturated hydrocarbons that contain at least one carbon-carbon triple bond (C≡C). They are the second most saturated class of hydrocarbons after alkanes.
Learning Outcomes
Describe the structure and properties of alkynes, alcohols and ethers;
Apply IUPAC nomenclature to alkynes, alcohols and ethers;
Understand and identify types of isomerism; and
Explain the reactivity and common reactions of alkynes, alcohols and ethers.
General Formula
The general formula for alkynes is CnH2n-2, where n is the number of carbon atoms in the molecule. For example, the molecular formula of ethyne (acetylene) is C2H2.
Structure
- The carbon atoms involved in the triple bond are sp-hybridized, resulting in a linear geometry around the triple bond with bond angles of 180°.
- The C≡C triple bond consists of one σ bond and two π bonds formed by the sideways overlap of p orbitals.
- Terminal alkynes have the formula RC≡CH, where R is an alkyl group. Internal alkynes have substituents on both acetylenic carbons.
Physical Properties
- Alkynes with 2-4 carbon atoms are gases at room temperature, 5-17 carbon atoms are liquids, and those with 18 or more carbon atoms are solids.
- Alkynes are colorless.
- Except for ethyne, which has characteristic smell, all members are odorless.
- They are less dense than water and insoluble in water but soluble in organic solvents.
- Alkynes are generally nonpolar molecules with little solubility in polar solvents, such as water. Solubility in nonpolar solvents, such as ether and acetone, is extensive.
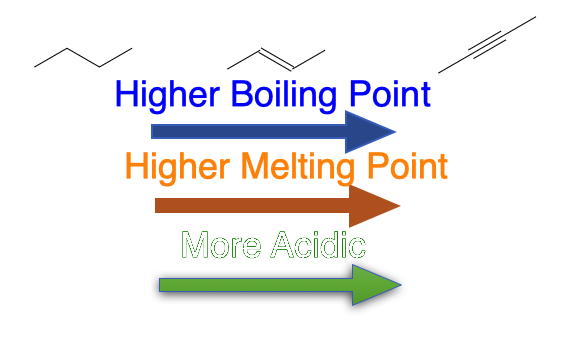
- The boiling point and melting point of alkynes increases as their molecular structure grows bigger. The boiling point increases with increase in their molecular mass.
- Compared to alkanes and alkenes, alkynes have a slightly higher boiling point, because the electric field of an alkyne, with its increased number of weakly held π electrons, is more easily distorted, producing stronger attractive forces between molecules.
Chemical Properties
- Alkynes are more reactive than alkanes due to the presence of the triple bond.
- Terminal alkynes are mildly acidic due to the acidity of the hydrogen atom attached to the sp-hybridized carbon.
- Alkynes undergo addition reactions, such as hydrogenation, halogenation, and hydration.
- Alkynes can participate in polymerization reactions.
Common Reactions
- Combustion: Alkynes undergo complete combustion in the presence of oxygen, producing carbon dioxide and water.
- Hydrogenation: Addition of hydrogen gas (H2) across the triple bond, catalyzed by transition metals.
- Halogenation: Addition of halogens (e.g., Cl2, Br2) across the triple bond.
- Hydration: Addition of water in the presence of an acid catalyst, forming aldehydes or ketones.
Isomerism
Alkynes with four or more carbon atoms can exhibit structural isomerism, where the triple bond can be positioned at different locations or have substituents on the carbon chain. Geometric isomerism is not possible in alkynes due to the linear geometry around the triple bond.
Applications
Acetylene (C2H2) is used as a fuel in oxyacetylene torches for cutting and welding metals. They are used as precursors in the synthesis of various organic compounds, such as acrylates. Alkynes are also employed as monomers in the production of certain polymers.
Naming Alkynes
Alkynes are named using the IUPAC (International Union of Pure and Applied Chemistry) nomenclature system. Here are the key steps for naming alkynes:
- Identify the longest carbon chain that includes both carbons of the triple bond. This will be the parent chain.
- Number the parent chain starting from the end closest to the triple bond. A 1-alkyne is called a terminal alkyne, while alkynes at any other position are called internal alkynes.
- Indicate the position of the triple bond by placing the number before the parent name. The parent name ends with "-yne" to indicate the presence of a triple bond.
- Name and number any substituents on the parent chain, placing them before the parent name in alphabetical order. Use prefixes like di-, tri-, tetra- for multiple substituents, but don't consider them for alphabetization.
- Combine the substituent names, numbers, and parent name to give the full IUPAC name. Separate numbers from letters with hyphens and use commas to separate numbers.
Examples
- Ethyne: The simplest alkyne, also known as acetylene (C2H2).
- Propyne: CH3C≡CH (1-propyne)
- 2-Butyne: CH3C≡CCH3 (2-butyne)
- 3-Hexyne: CH3CH2C≡CCH2CH3 (3-hexyne)
- 4-Ethyl-2,5-octadiyne: CH3CH2C≡CCH(C2H5)C≡CCH3
Some key points:
- Alkynes cannot exhibit cis-trans isomerism due to the linear geometry around the triple bond.
- Substituents containing a triple bond are called alkynyl groups (e.g. -C≡CH is ethynyl).
- Molecules with both double and triple bonds are called alkenynes. The chain is numbered from the end closest to the multiple bonds, with the suffixes "-ene" and "-yne" in alphabetical order.
By following these rules, the IUPAC name uniquely identifies the structure of an alkyne. The systematic naming allows for clear communication of alkyne structures in organic chemistry.
Alkynes in Agrochemicals
Alkynes are relevant to agriculture in several ways, particularly in the development of agrochemicals and as markers for studying plant-animal interactions.
Many alkyne derivatives play important roles in the control of weeds, insects, and fungal diseases affecting crops. Alkynes serve as key intermediates in the preparation of various agrochemicals due to their high reactivity and ability to undergo diverse chemical reactions.
A specific examples is acetylene is used as a fuel in oxyacetylene torches for burning and welding purposes in agriculture.
As research in alkyne chemistry continues to advance, it is likely that more applications in agriculture will emerge. The structural diversity of alkyne derivatives and their versatility in organic synthesis suggest that they may find increasing use in the development of novel agrochemicals and other agricultural products.








.png)
.png)
.png)
Comments
Post a Comment