003 Alkenes
Hydrocarbons - Alkenes
Alkenes are a class of unsaturated hydrocarbons characterized by the presence of at least one carbon-carbon double bond (C=C). They are significant in organic chemistry due to their unique properties and reactivity. Here’s a detailed overview of alkenes, including their general formula, physical and chemical properties, common reactions, structure, and isomerism.
Learning Outcomes
Identify and illustrate common organic molecules and functional groups;
- Understand basic concepts of organic chemistry;
- Describe structure, properties, and isomerism in alkenes;
- Apply nomenclature rules for alkenes; and
- Explain hybridization, molecular geometry in organic compounds, and the reactivity and common reactions of alkenes.
General Formula
The general formula for alkenes is CnH2n, where n is the number of carbon atoms. This formula indicates that alkenes have two fewer hydrogen atoms than the corresponding alkanes (which have the general formula CnH2n+2 due to the presence of the double bond.
Structure
Alkenes are a class of hydrocarbons characterized by the presence of at least one carbon-carbon double bond (C=C), which distinguishes them from alkanes. This double bond consists of one sigma (σ) bond and one pi (π) bond, resulting from the sidewise overlap of p orbitals. The presence of the double bond imparts a planar geometry around the carbon atoms involved, with bond angles of approximately 120 degrees due to sp² hybridization. This hybridization allows each carbon atom to form three sigma bonds: two with adjacent carbon atoms and one with a hydrogen atom or another substituent. Alkenes can exist as straight-chain or branched structures and exhibit geometric isomerism, where the spatial arrangement of substituents around the double bond can lead to distinct cis and trans configurations, influencing their physical and chemical properties.
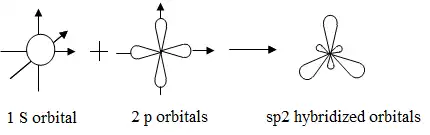 |
| sp² hybridized carbon atoms result in a trigonal planar geometry with bond angles of approximately 120°. |
Physical Properties
Alkenes possess distinct physical properties that differentiate them from alkanes and other hydrocarbons. Generally, they are colorless and have a mild, pleasant odor, with low boiling and melting points that increase with molecular weight due to stronger Van der Waals forces. Alkenes are typically liquid at room temperature for smaller molecules, while larger alkenes can be waxy solids.
Their unsaturation from the carbon-carbon double bond makes them more reactive than alkanes, affecting their solubility; alkenes are nonpolar and insoluble in water, but they readily dissolve in nonpolar organic solvents.
Additionally, alkenes exhibit geometric isomerism, which can lead to variations in boiling points and other physical properties based on the spatial arrangement of substituents around the double bond.
Overall, the physical properties of alkenes are influenced by their molecular structure and the presence of the double bond, making them important in both industrial applications and organic synthesis.
Naming Alkenes
Alkenes are named using a systematic approach based on the IUPAC (International Union of Pure and Applied Chemistry) nomenclature rules. Here’s a detailed guide on how to name alkenes:
- Identify the Longest Carbon Chain: Find the longest continuous carbon chain that contains the double bond. This chain will serve as the parent hydrocarbon.
- Number the Carbon Atoms: Number the carbon atoms in the chain starting from the end closest to the double bond. This ensures that the double bond receives the lowest possible number.
- Indicate the Position of the Double Bond: The position of the first carbon in the double bond is indicated by a number placed before the suffix "-ene." For example, if the double bond starts at carbon 2 in a five-carbon chain, the name would be "pent-2-ene."
- Name the Substituents: If there are any substituents (alkyl groups or other functional groups) attached to the parent chain, name them and indicate their positions using numbers. List the substituents in alphabetical order, regardless of their position numbers.
- Write the Full Name: Combine the substituent names and their positions with the parent name, including the position of the double bond. For example, if there is a methyl group on carbon 3 of a pentene, the name would be "3-methylpent-2-ene."
- Multiple Double Bonds: If the alkene contains more than one double bond, use the suffixes "-diene" for two double bonds and "-triene" for three double bonds, indicating the positions of each double bond. For example, "1,3-butadiene" indicates double bonds at positions 1 and 3.
Example Names
- Ethene (C2H4): The simplest alkene, commonly known as ethylene.
- Propene (C3H6): The three-carbon alkene.
- But-1-ene (C4H8): A four-carbon alkene with the double bond starting at carbon 1.
- 2-Methylbut-2-ene (C5H10): A five-carbon alkene with a methyl substituent on carbon 2 and a double bond starting at carbon 2.
- 1,3-Hexadiene (C6H10): A six-carbon alkene with double bonds at positions 1 and 3.
Cycloalkenes
For cycloalkenes, the naming is slightly different:
- The double bond is always between carbon 1 and carbon 2, so it is not necessary to indicate the position of the double bond in the name.
- Number the ring so that the first substituent receives the lowest number. For example, "3-Methylcyclohexene" indicates a methyl group on carbon 3 of the cyclohexene.
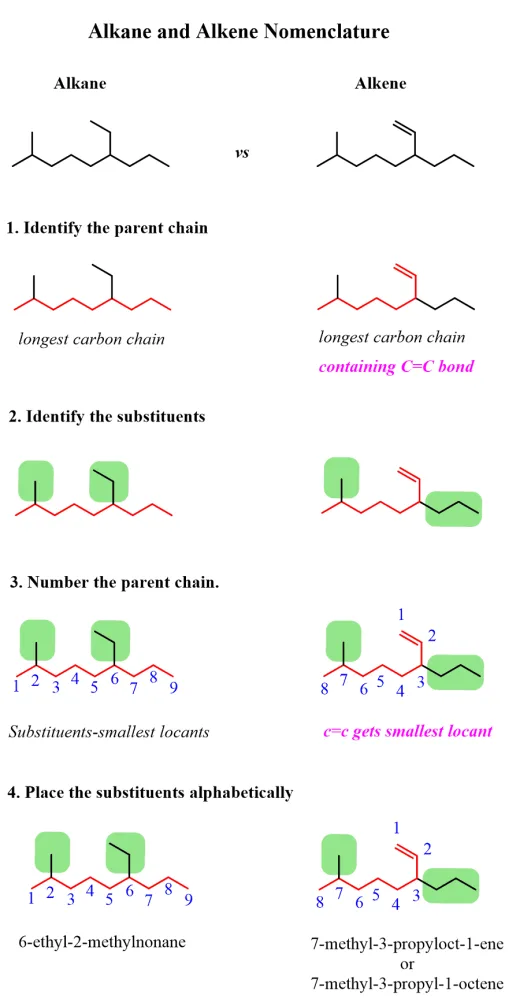 |
| Naming alkanes vs. alkenes |
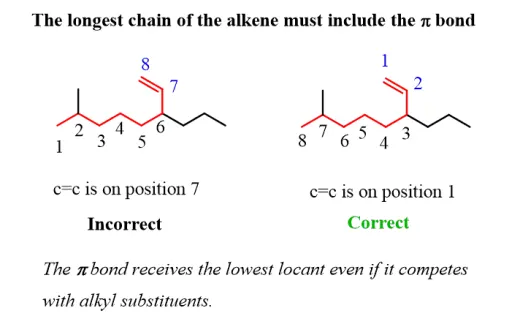 |
| The longest chain should include the double (sigma) bond. Numbering the carbon atom should give the lowest number to the double bond. |
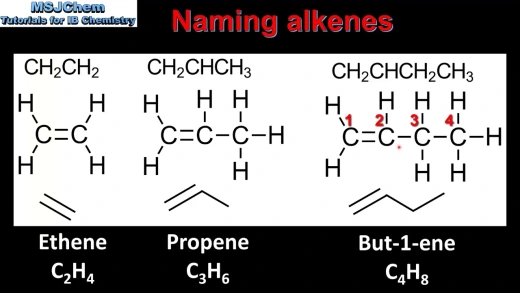 |
| Example of alkenes |
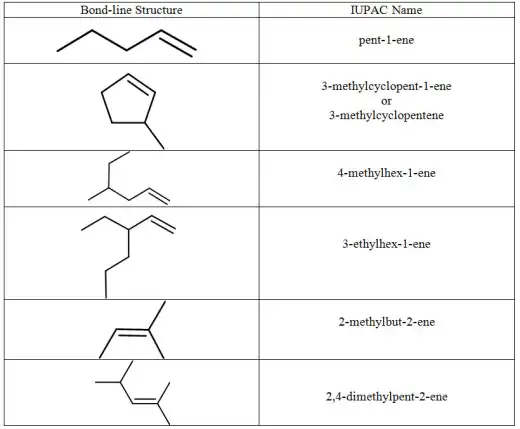 |
| More examples of alkenes |
Isomerism
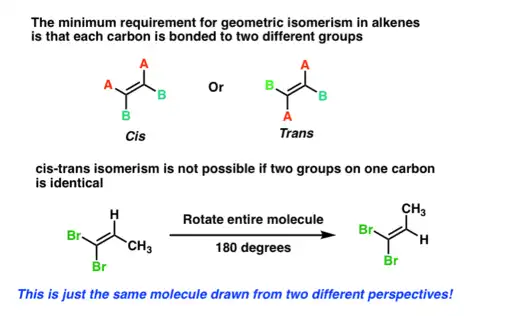
Alkenes can exhibit geometric (cis-trans) isomerism due to the restricted rotation around the double bond:
Cis Isomers: The substituents on the double bond are on the same side.
Trans Isomers: The substituents are on opposite sides.
Additionally, alkenes can exhibit structural isomerism, where the connectivity of the carbon skeleton varies.
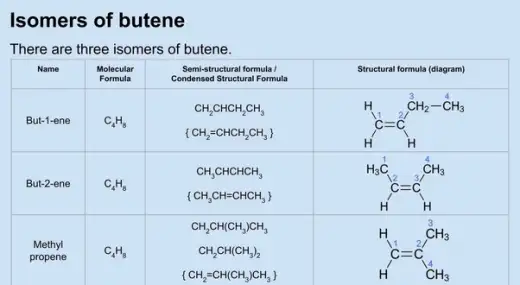 |
| Structural isomerism in alkenes |
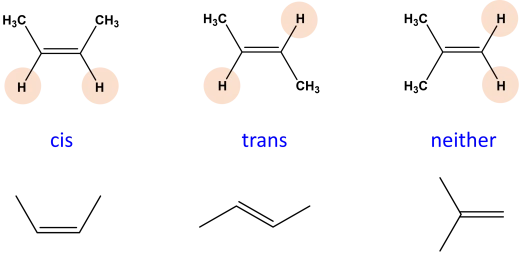 |
| Example of cis, trans and non-isomer |
Chemical Properties
- Reactivity: Alkenes are more reactive than alkanes due to the presence of the double bond, which can participate in various chemical reactions.
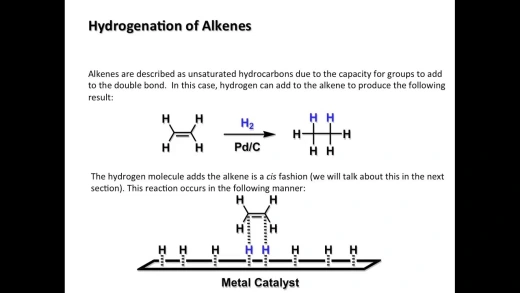
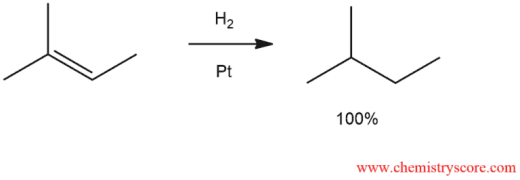
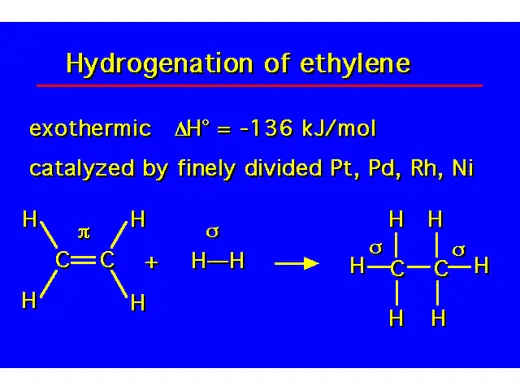
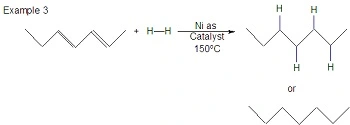
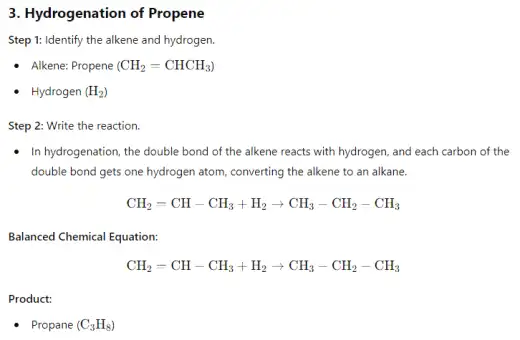
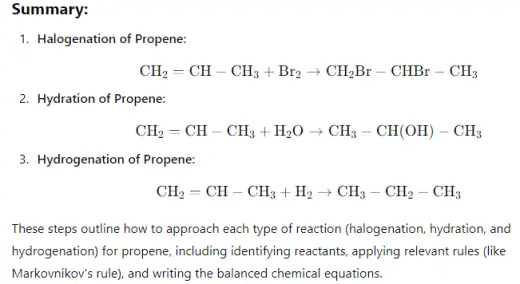
- Hydrogenation of alkenes is a chemical reaction where hydrogen (H₂) is added across the double bond of an alkene, converting it into an alkane. This reaction typically requires a metal catalyst, such as platinum (Pt), palladium (Pd), or nickel (Ni), and is conducted under pressure. The process involves breaking the double bond and adding one hydrogen atom to each of the carbon atoms involved in the bond, effectively saturating the molecule and removing any unsaturation. The result is a more stable, single-bonded alkane. Hydrogenation is widely used in industrial applications, such as the production of margarine from vegetable oils.
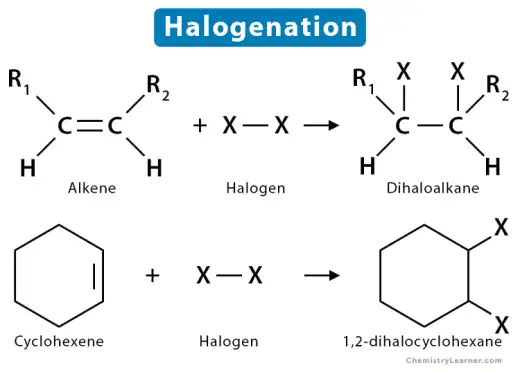
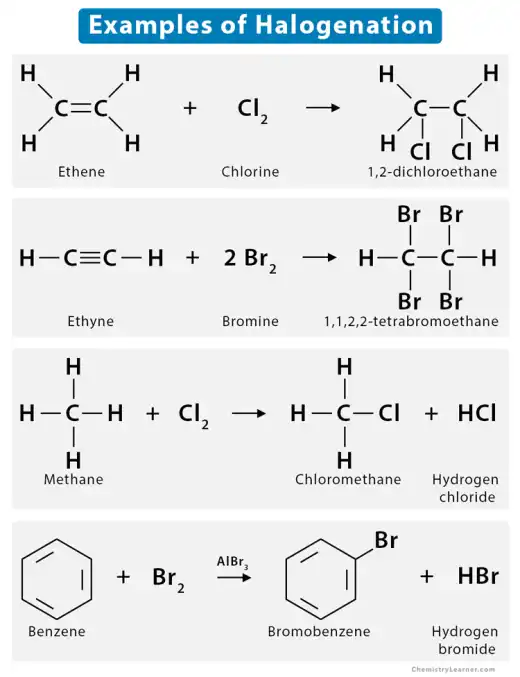
- Halogenation of alkenes is a reaction where a halogen, such as chlorine (Cl₂) or bromine (Br₂), is added across the double bond of an alkene, forming a dihaloalkane. The reaction occurs via an electrophilic addition mechanism, where the alkene's double bond attacks the halogen molecule, creating a cyclic halonium ion intermediate. The halide ion then opens the ring, resulting in the addition of one halogen atom to each carbon of the former double bond. This reaction is typically fast, exothermic, and produces vicinal (neighboring) dihalides. Halogenation is a common method for testing unsaturation in organic compounds.
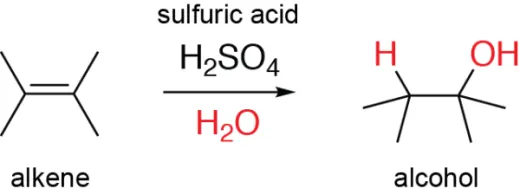
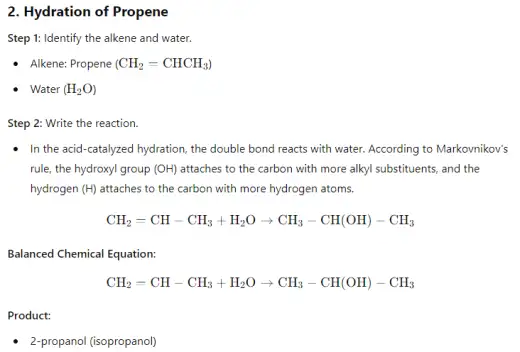
- Hydration of alkenes is a chemical reaction where water (H₂O) is added across the double bond of an alkene, converting it into an alcohol. This reaction typically requires an acid catalyst, such as sulfuric acid (H₂SO₄), and follows Markovnikov's rule, where the hydrogen atom from water attaches to the carbon with the most hydrogen atoms, and the hydroxyl group (OH) attaches to the other carbon. The process involves the formation of a carbocation intermediate, followed by the nucleophilic attack of water. Hydration is a key reaction in organic chemistry for synthesizing alcohols from alkenes.
- Polymerization of alkenes is a chemical process where small alkene molecules (monomers) join together to form long chains called polymers. This process involves the breaking of the double bonds in the alkene monomers, allowing them to link together in a repeating pattern. The reaction is typically initiated by heat, pressure, or a catalyst, and can proceed via different mechanisms, such as free-radical polymerization or coordination polymerization. Common products of alkene polymerization include polyethylene and polypropylene, which are widely used in plastics, packaging, and textiles.

.png)
.png)
.png)
Comments
Post a Comment