002 Alkanes
Hydrocarbons - Alkanes
Alkanes
Alkanes play a vital role in agriculture as energy sources, raw materials for agrochemicals, solvents, lubricants, and in research related to environmental impact. Their versatility and importance in various agricultural applications highlight their contribution to enhancing productivity and sustainability in farming practices.
Learning Outcomes
Identify and illustrate common organic molecules and functional groups;
- Understand basic concepts of organic chemistry;
- Describe structure, properties, and isomerism in alkanes;
- Apply nomenclature rules for alkanes; and
- Explain hybridization, molecular geometry in organic compounds, and the reactivity and common reactions of alkanes.
General Formula
Alkanes are the simplest class of hydrocarbons, consisting entirely of carbon (C) and hydrogen (H) atoms connected by single covalent bonds. Their structure is characterized by the following key features:
Alkanes follow the general formula CnH2n+2, where n is the number of carbon atoms. For example:
- Methane: C1H4 (CH₄)
- Ethane: C2H6 (C₂H₆)
- Propane: C3H8 (C₃H₈)
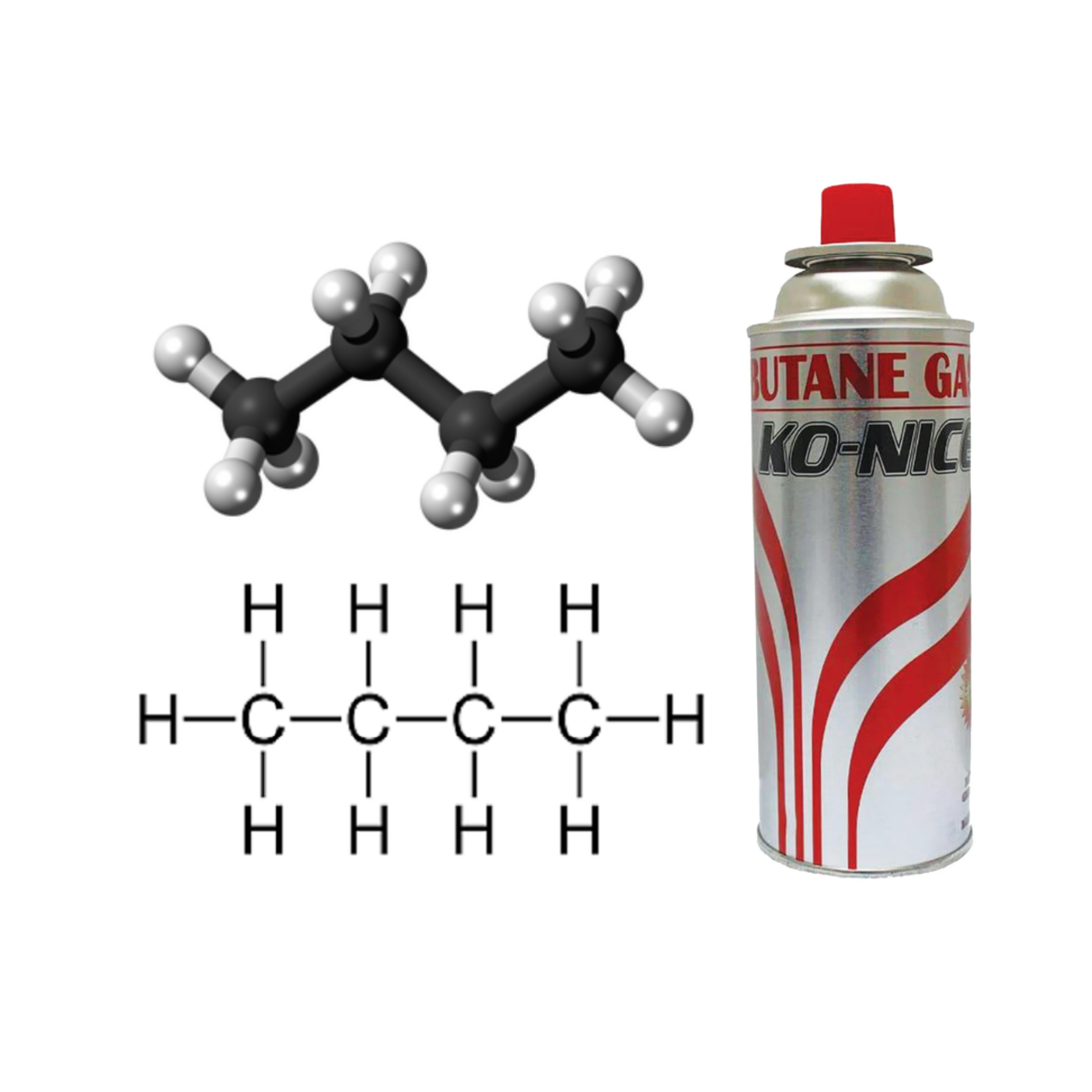
- Butane: C4H10 (C₄H₁₀)
- Pentane: C5H12 (C₅H₁₂)
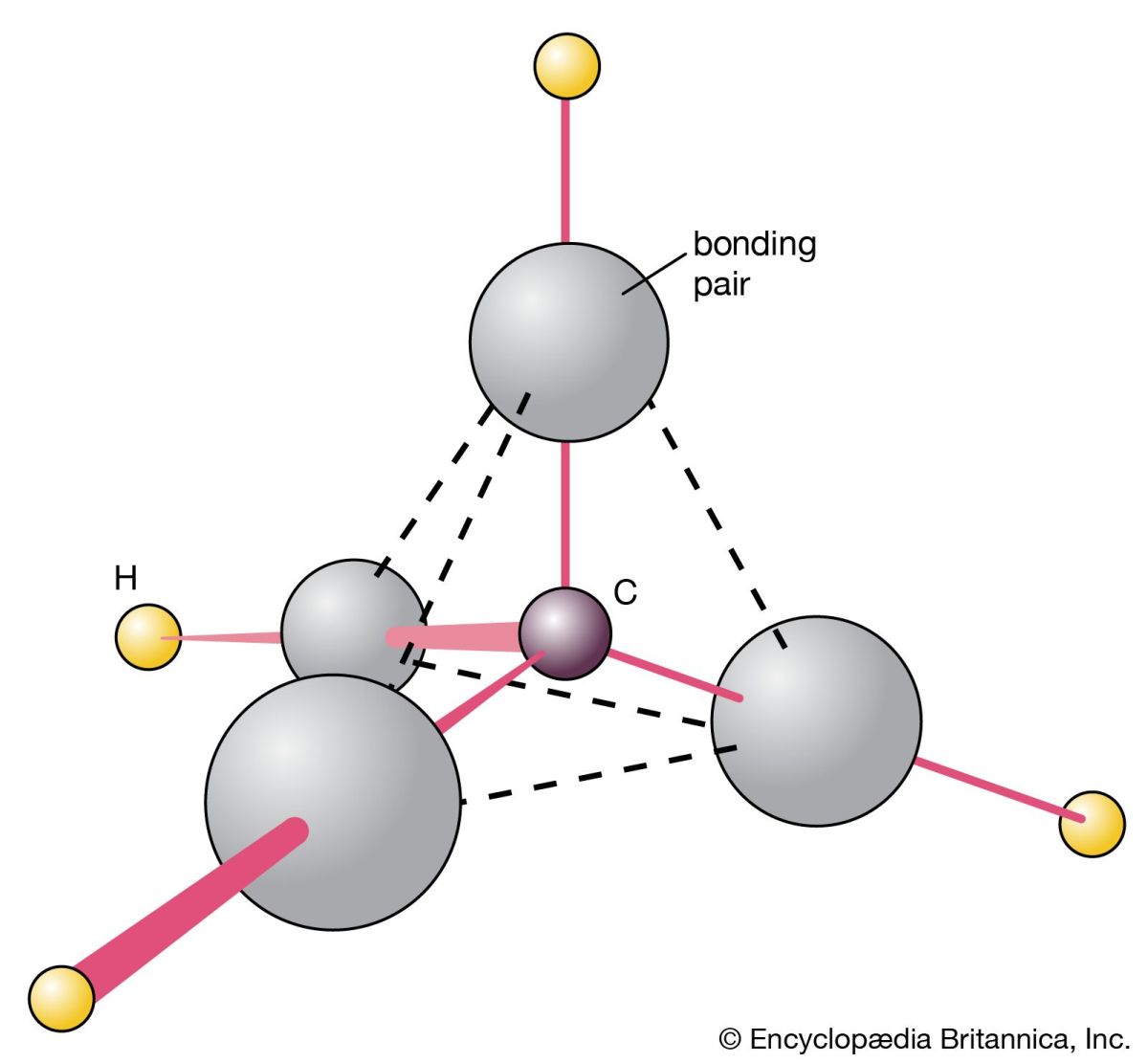 |
| Methane is gas that is found in small quantities in the atmosphere. It is the simplest hydrocarbon, consisting of one carbon atom and four hydrogen atoms. It is also a powerful greenhouse gas. |
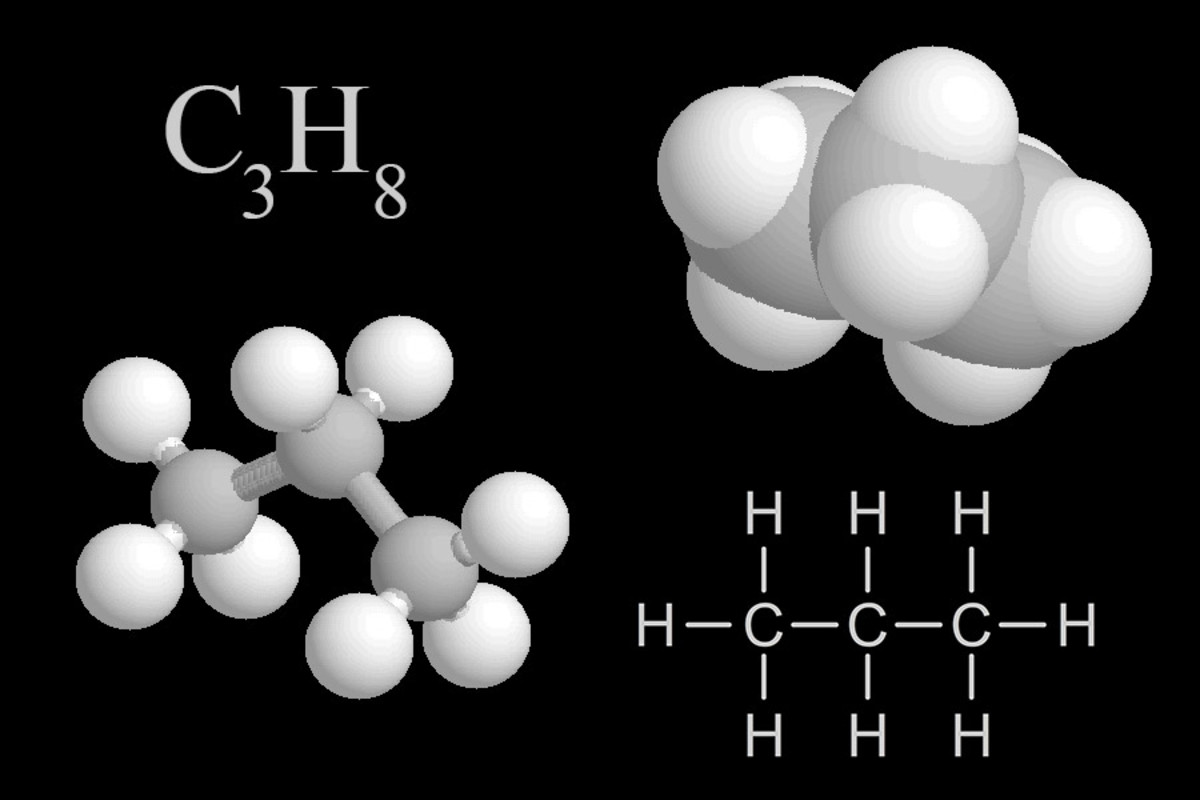 |
| Propane is a type of gas, commonly burned for heating, cooking, and as a fuel for engines. |
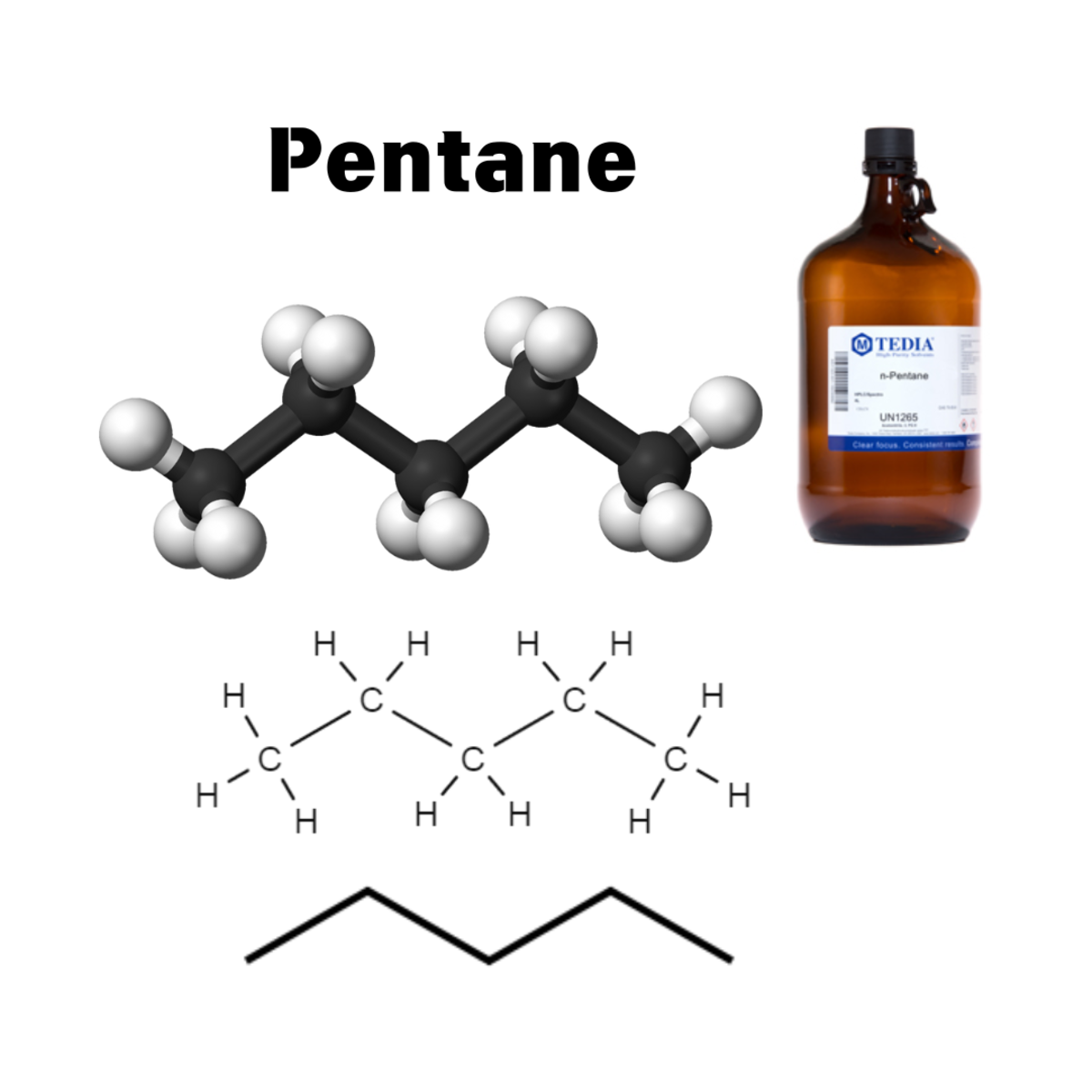 |
| Carbon chains are often depicted as crooked in chemical structures, but this is primarily a simplification for visual representation. In reality, carbon chains are constantly moving and flexible. |
Structure
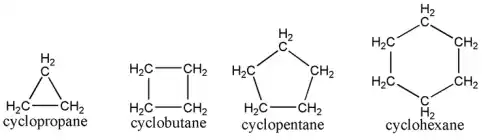
Alkanes consist solely of sigma (σ) bonds, formed by the head-on overlap of atomic orbitals, with each carbon atom creating four single bonds with other carbon or hydrogen atoms. The carbon atoms in alkanes are sp³ hybridized, resulting in a tetrahedral geometry with bond angles of approximately 109.5°. Alkanes can be straight-chain (normal) or branched, with straight-chain alkanes having a linear arrangement of carbon atoms, while branched alkanes feature additional carbon atoms attached to the main chain. Additionally, alkanes can form cyclic structures known as cycloalkanes, where carbon atoms are arranged in a ring, following the formula CnH2n due to the loss of two hydrogen atoms for each bond in the ring.
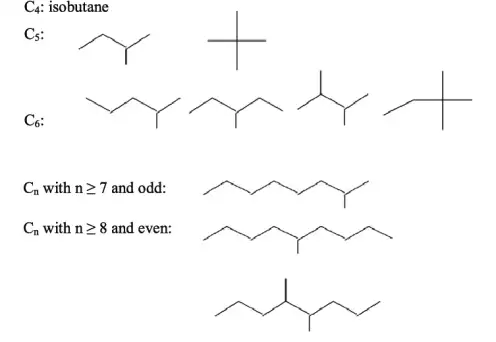 |
| The various ways carbon atoms can be arranged in branched chain structures within alkanes |
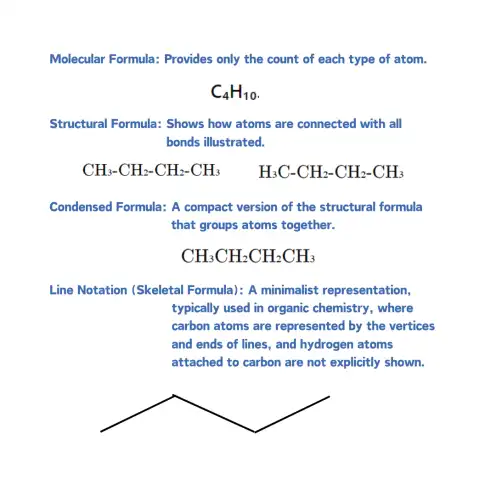 |
| Different ways organic molecules are represented |
Physical Properties of Alkanes
Alkanes exhibit varying states at room temperature based on their carbon chain length: those with 1 to 4 carbon atoms (e.g., methane, ethane) are gases, 5 to 17 carbon atoms (e.g., pentane, hexane) are typically liquids, and those with 18 or more carbon atoms (e.g., hexadecane) are solids. Generally colorless and odorless, alkanes are suitable for applications where these traits are beneficial. Their boiling and melting points increase with molecular weight due to stronger Van der Waals forces, with straight-chain alkanes having higher boiling points than their branched isomers due to greater surface area. Alkanes are nonpolar and insoluble in water, making them hydrophobic, but they dissolve in nonpolar organic solvents. Additionally, alkanes typically have lower densities than water, allowing them to float, with density increasing as the number of carbon atoms in the chain rises.
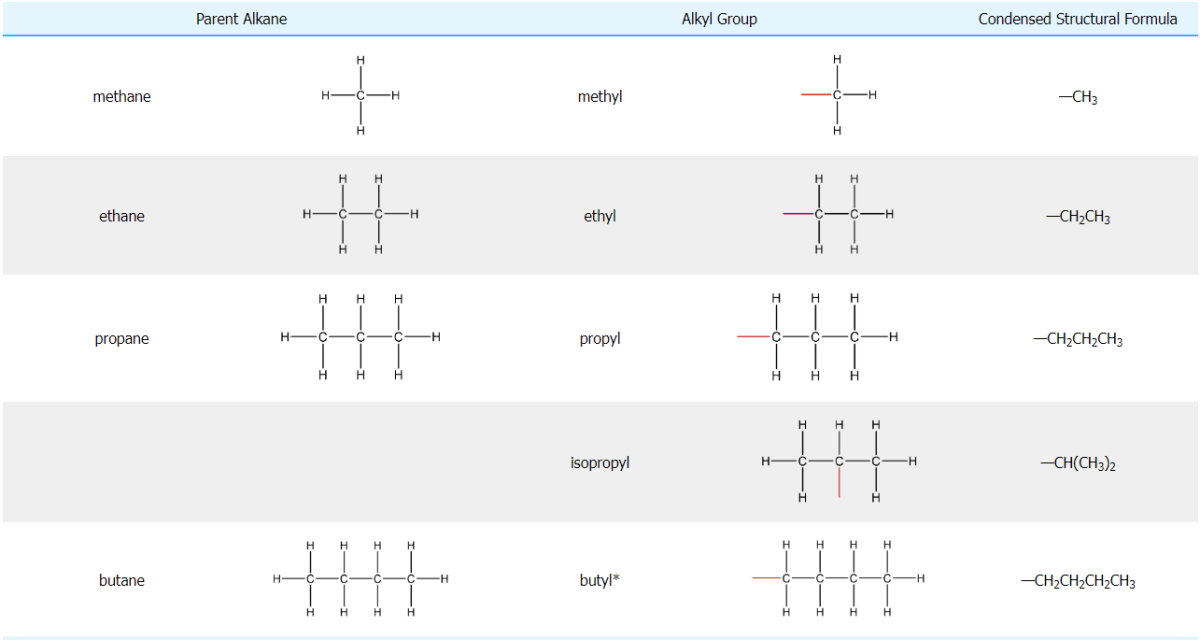
Naming Alkanes
Alkanes are named using the IUPAC (International Union of Pure and Applied Chemistry) nomenclature system. The key steps are:
Identify the longest continuous chain of carbon atoms. This is known as the parent chain or root name. The suffix "-ane" is added to the appropriate numerical prefix to name the parent chain.
- Number the carbon atoms in the parent chain starting from the end closest to a substituent or branch. The substituents are numbered based on the lowest possible numbers.
- Name and locate the substituents alphabetically. Common substituents include alkyl groups like methyl (-CH3), ethyl (-CH2CH3), propyl (-CH2CH2CH3), etc.
- If there are multiple substituents, use numerical prefixes like di-, tri-, tetra- to indicate the number of substituents. The position of each substituent is indicated by a number.
- The substituent names are placed before the parent name, separated by a hyphen. If there are multiple substituents, commas separate the numbers.
Some examples:
- Methane (CH4)
- Ethane (CH3CH3)
- Propane (CH3CH2CH3)
- 2-Methylpropane (CH3CH(CH3)CH3)
- 2,2,4-Trimethylpentane (CH3C(CH3)2CH2CH(CH3)2)
Straight-chain alkanes can be indicated with the prefix "n-" (e.g. n-hexane) to distinguish them from branched isomers. Certain branched alkanes also have common names using prefixes like iso-, sec-, tert-, and neo-.
IUPAC Names of the First 20 Parent Alkanes
Number of carbon atoms | Name |
|---|---|
1 | methane |
2 | ethane |
3 | propane |
4 | butane |
5 | pentane |
6 | hexane |
7 | heptane |
8 | octane |
9 | nonane |
10 | decane |
11 | undecane |
12 | dodecane |
13 | tridecane |
14 | tetradecane |
15 | pentane |
16 | hexadecane |
17 | heptadecane |
18 | octadecane |
19 | nonadecane |
20 | icosane |
Chemical Properties of Alkanes
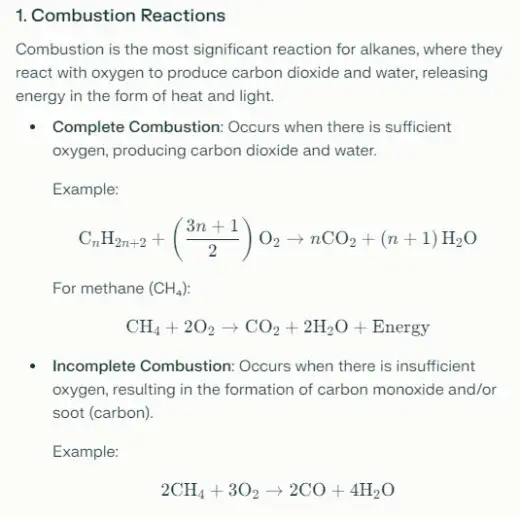
- Combustion: Alkanes readily undergo combustion reactions in the presence of oxygen, producing carbon dioxide and water along with the release of energy. This reaction is exothermic and is a primary reason alkanes are used as fuels.
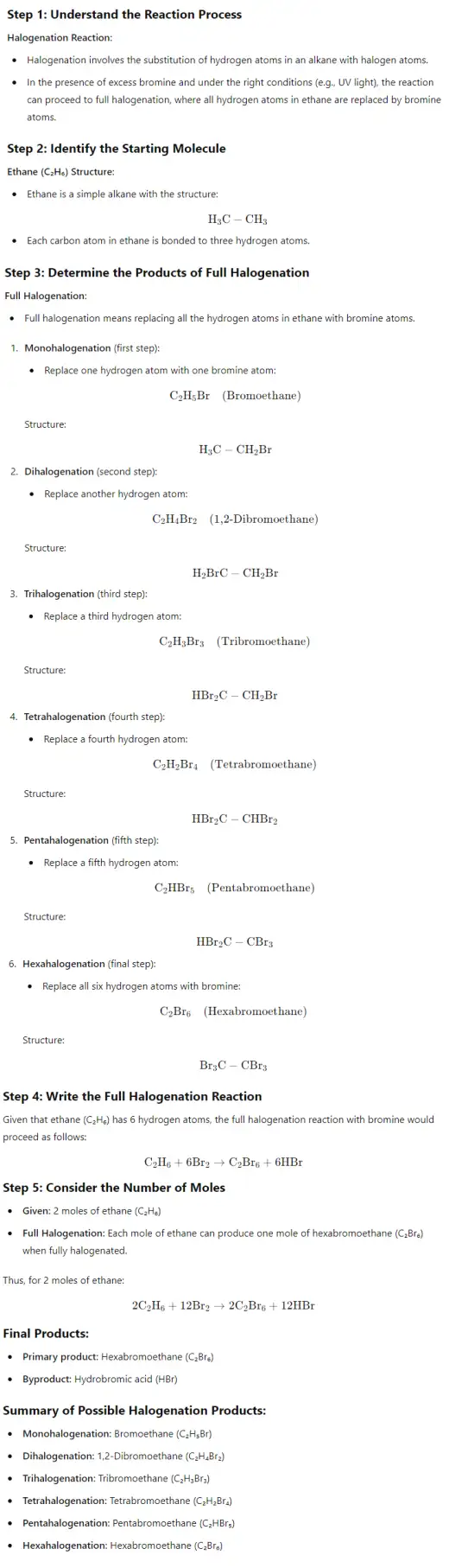
- Halogenation: Alkanes can react with halogens (e.g., chlorine, bromine) in the presence of ultraviolet light, leading to substitution reactions where hydrogen atoms are replaced by halogen atoms.
- Reactivity: Alkanes are generally less reactive than other organic compounds due to the stability of their C-C and C-H bonds. Their reactions are primarily limited to combustion and halogenation under specific conditions.
- Isomerization: Alkanes can undergo isomerization, where straight-chain alkanes are converted into branched-chain isomers. This process often requires a catalyst, such as aluminum chloride (AlCl₃).
- Cracking: Higher alkanes can be broken down into smaller alkanes and alkenes through a process called cracking, which involves heating the alkanes to high temperatures. This reaction is important in petroleum refining.
- Pyrolysis: Pyrolysis is a thermal decomposition reaction that occurs at high temperatures, breaking down larger alkanes into smaller hydrocarbons.
Isomerism
As the number of carbon atoms increases, alkanes can exhibit structural isomerism, where compounds have the same molecular formula but different structural arrangements. For example, butane (C₄H₁₀) has two isomers: n-butane (a straight chain) and isobutane (a branched chain).
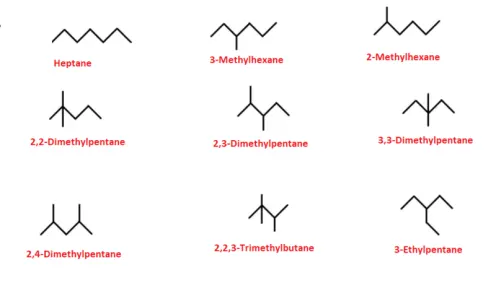 |
| All the possible isomers of alkane having 7 carbon atoms and their IUPAC names |
Essentially, alkanes are characterized by their saturated nature, single C-C bonds, tetrahedral geometry, and the ability to form various structural isomers. Their simple structure makes them fundamental to the study of organic chemistry and serves as the backbone for more complex organic molecules.
Problem Set
1. Write the balanced chemical equation for the complete combustion of dodecane, hexadecane, and heptadecane.
2. Given 7 moles of 2-methylpropane and excess chlorine(Cl₂), write the possible halogenation products if the reaction goes to full halogenation. Provide the structure of each product.
3. List all possible isomers of octane. Use line notation to depict each isomer and provide its name.

.png)
.png)
.png)
Comments
Post a Comment