001 Introduction to Organic Chemistry
Organic chemistry plays a crucial role in agriculture, significantly impacting crop production, protection, and sustainability. It is integral to modern agriculture, influencing everything from soil management to pest control. Its applications not only enhance crop yields but also contribute to sustainable agricultural practices that are essential for meeting future food demands.
Learning Outcomes
Describe the importance and applications of organic chemistry in agriculture;
Identify and illustrate common organic molecules and functional groups;
- Understand basic concepts of organic chemistry;
- Describe structure, properties, and isomerism in alkanes and alkenes;
- Apply nomenclature rules for alkanes and alkynes; and
- Explain hybridization, molecular geometry in organic compounds, and the reactivity and common reactions of alkanes and alkenes.
Agriculture and Organic Chemistry
Organic chemistry plays a crucial role in agriculture by enhancing soil fertility through the formulation of fertilizers, which can be either organic—derived from natural sources like manure and compost—or inorganic, made from synthetic compounds such as ammonium nitrate.
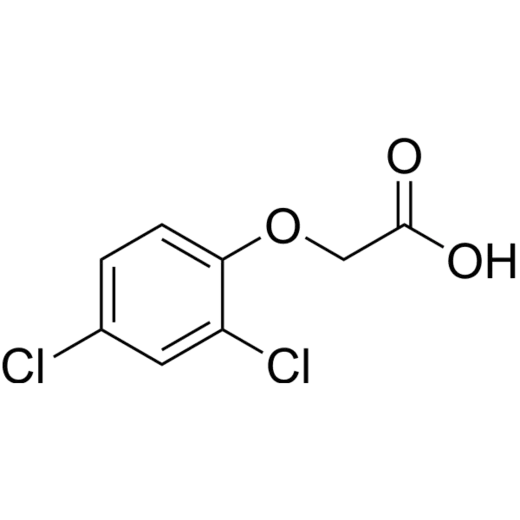
Additionally, organic chemistry is essential in developing pesticides, including herbicides and insecticides, that protect crops from pests and diseases, exemplified by the herbicide 2,4-dichlorophenoxyacetic acid. Furthermore, it supports crop protection strategies against fungal pathogens, insect pests, and weeds, ensuring high yields to meet the demands of a growing global population.
As sustainability becomes increasingly important, organic chemistry also contributes to creating biodegradable and less toxic agricultural chemicals, focusing on methods that minimize environmental impact while maintaining productivity, such as synthesizing target-specific chemicals that safely degrade in the environment.
 |
| 2,4-D can be found in lawn herbicide mixtures such as "Weed B Gon MAX", "PAR III", "Trillion", "Tri-Kil", "Killex" and "Weedaway Premium 3-Way XP Turf Herbicide". |
Introduction to Organic Chemistry
Organic chemistry is a branch of chemistry focused on the study of carbon-containing compounds, encompassing their structure, properties, composition, reactions, and synthesis. Initially, organic chemistry was concerned primarily with substances derived from living organisms, but it has since expanded to include synthetic compounds such as plastics and pharmaceuticals.
Organic chemistry focuses on carbon-based compounds, typically consisting of carbon bonded to hydrogen, oxygen, nitrogen, sulfur, and halogens, allowing for diverse molecular structures such as chains, rings, and complex arrangements.
The field encompasses the study of both the physical and chemical properties of these compounds, including their reactivity and reaction mechanisms, which facilitate the synthesis of products like natural substances, drugs, and polymers.
Organic chemistry is essential across various industries, including pharmaceuticals, biotechnology, consumer products, and industrial chemistry, where it aids in developing medications, genetically modified crops, cosmetics, and plastics.
Furthermore, it serves as a foundational discipline for biochemistry, medicinal chemistry, and materials science, providing vital insights into biological processes and technological advancements. Overall, organic chemistry is crucial for understanding chemical compounds and their applications in everyday life and industry.
Basic Concepts in Organic Chemistry
Organic chemistry encompasses their structure, properties, reactions, and synthesis. Carbon's unique ability to form four covalent bonds allows for diverse molecular structures, with functional groups determining reactivity.
Isomerism occurs when compounds have the same molecular formula but different structures or arrangements. Using the IUPAC nomenclature, organic compounds are named systematically based on the longest carbon chain, functional groups, and branching.
Covalent bonding, including single, double, and triple bonds, is crucial, with reactivity and mechanisms involving nucleophiles, electrophiles, and step-by-step descriptions. Physical properties like boiling point, solubility, and density are influenced by molecular structure and functional groups.
Hybridization, particularly sp3, sp2, and sp, explains the bonding behavior of atoms in organic molecules, with tetrahedral, trigonal planar, and linear geometries, respectively. Understanding these concepts is essential for applying organic chemistry to diverse fields, from agriculture to materials science.
Common Organic Molecules and Functional Groups
Organic compounds are a diverse class of molecules crucial to life, encompassing various functional groups with numerous applications across multiple fields.
Hydrocarbons, the simplest organic compounds, include alkanes (saturated, e.g., methane), alkenes (unsaturated with double bonds, e.g., ethylene), and alkynes (unsaturated with triple bonds, e.g., acetylene).
Alcohols feature a hydroxyl (-OH) group (e.g., ethanol), while carboxylic acids contain a carboxyl (-COOH) group (e.g., acetic acid).
Aldehydes and ketones both possess a carbonyl (C=O) group, differing in its position (e.g., formaldehyde and acetone, respectively).
Esters, formed from carboxylic acids and alcohols, are known for their pleasant odors (e.g., ethyl acetate).
Amines, containing nitrogen with attached alkyl or aryl groups, can be primary, secondary, or tertiary (e.g., methylamine).
Functional groups such as hydroxyl, carbonyl, carboxyl, amino, sulfhydryl, and phosphate dictate the chemical reactivity and physical properties of these compounds, making them essential in biochemistry, medicine, materials science, and everyday products.

.png)
.png)
.png)
Comments
Post a Comment